��Ŀ����
����Ŀ����������ɸ�������ɺ����ʽ��з���
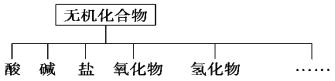
(1)ͼ����ʾ�����ʷ������������________��
(2)��Na��K��H��O��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��Тڢۢ��档
������� | �� | �� | �� | ������ | �⻯�� |
��ѧʽ | ��HCl ��____ | ��________ ��Ba(OH)2 | ��Na2CO3 ��______ | ��CO2 ��Na2O | ��NH3 ��H2O2 |
(3)д����ת��Ϊ�ݵĻ�ѧ����ʽ��___________��
(4)ʵ�����Ʊ��߳���______��______��Ӧ�����������ķ�����____________��
���𰸡���״���෨ H2SO4 NaOH K2SO4 CO2+2NaOH�TNa2CO3+H2O CaCO3 ϡ���� ͨ�����ʯ��ˮ��������ǣ������ɵ�����ΪCO2
��������
(1)��״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô����ֳ�֦Ѿ��
(2)��������������ȫ���������ӵĻ������������������ȫ��Ϊ���������ӣ��ε������������Ϊ�������ӣ�������Ϊ������ӣ�
(3)CO2��NaOH��Ӧת��ΪNa2CO3��
(4)ʵ�����Ʊ�������̼���� CaCO3��ϡ���ᷴӦ�����������ķ����� ͨ�����ʯ��ˮ���Ƿ����ǡ�
(1)��״���෨��һ�ֺ�����ķ��෨�����ղ�Σ�һ��һ�����֣�����һ�ô�������Ҷ��֦���ˡ�����ͼʾ����������״ͼ����Ϊ��״���෨��
(2)�������ˮ��Һ�е������������ȫ��Ϊ�����ӵĻ������H2SO4����������������ȫ��Ϊ���������ӣ���NaOH���ε������������Ϊ�������ӣ�������Ϊ������ӣ���K2SO4���ʴ�Ϊ����H2SO4����NaOH����K2SO4��
(3)CO2��NaOH��Ӧת��ΪNa2CO3���仯ѧ����ʽΪ��CO2+2NaOH�TNa2CO3+H2O��
(4)ʵ�����Ʊ�������̼���� CaCO3��ϡ���ᷴӦ�����������ķ����� ͨ�����ʯ��ˮ��������ǣ������ɵ�����ΪCO2��

����Ŀ��ij����С����õ���̷ۣ���Ҫ�ɷ�ΪMn��������Fe��Ni��Pb��P��Si��Ԫ�صĵ��ʻ��仯���Ϊԭ���Ʊ��ߴ��Ȼ��̡�
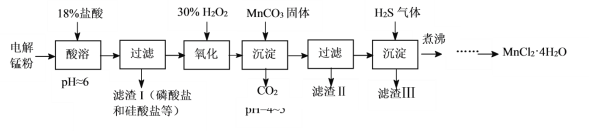
��֪����Mn��һ�ֱ�Fe���õĽ�����
��H2S������н�ǿ�Ļ�ԭ�ԣ��磺H2S+H2O2=S��+2H2O��
����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ1.0mol��L-1���㣩
Fe3+ | Fe2+ | Mn2+ | Ni2+ | Pb2+ | |
��ʼ������pH | 1.1 | 5.8 | 8.6 | 6.7 | 8.0 |
������ȫ��pH | 3.2 | 8.8 | 10.1 | 9.2 | 8.8 |
��1������ʱ����Ҫ�����μӵ�ԭ����___��
��2������MnCO3����ʱ������Ӧ�����ӷ���ʽΪ___��MnCO3����Ҳ�����������ʴ���___������д��ţ�
A.MnO B.MnSO4 C.Mn(OH)2 D.MnCl2
��3��ͨ��H2S�����ʹPb2+��Ni2+��������������磺H2S(aq)+Pb2+(aq)![]() PbS(s)+2H+(aq)���÷�Ӧ��ƽ�ⳣ��K=___[��Ka1(H2S)��Ka2(H2S)��Ksp(PbS)��ʾ]��
PbS(s)+2H+(aq)���÷�Ӧ��ƽ�ⳣ��K=___[��Ka1(H2S)��Ka2(H2S)��Ksp(PbS)��ʾ]��
��4����е�Ŀ����___��
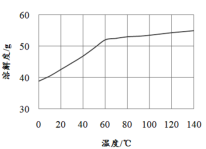
��5����֪MnCl2��4H2O��106��ʱʧȥһ���ӽᾧˮ��198��ʧȥȫ���ᾧˮ���벹����������к����Һ��øߴ���>99.99%��MnCl2��4H2O���壨MnCl2���ܽ��������ͼ��ʾ����ʵ�鷽��������к����Һ��ȴ�����£�___��ʵ������ʹ�õ��Լ��ǣ�30%H2O2����
����Ŀ���⼰�仯�����ںϳ�ɱ������ҩ��ȷ�����й㷺��;���ش��������⣺
��1����֪��
H2(g)+I2(g)=2HI(g) ��H=QkJ��mol-1
H2(g)+I2(s)=2HI(g) ��H=��26.48kJ��mol-1
I2(g)=I2(s) ��H=-37.48kJ��mol-1
��ѧ�� | I��I | H��I |
����/��kJ��mol-1�� | 151 | 299 |
�����ǽ�1mol�������AB����Ϊ������̬ԭ��A��B����Ҫ����������λΪkJ��mol-1��
��Q=____kJ��mol-1��
��H��H����Ϊ____kJ��mol-1��
��2��716Kʱ���ں����ܱ������а����ʵ���֮��1��1����H2(g)��I2(g)��������������е⻯������ʵ��������뷴Ӧʱ��Ĺ�ϵ��ͼ��

������Ӧ��ʼʱ�����������ѹΪpkPa����Ӧ��ǰ20min�ڵ�I2(g)ƽ������![]() (I2)=___kPa��min-1���ú�p��ʽ�ӱ�ʾ����
(I2)=___kPa��min-1���ú�p��ʽ�ӱ�ʾ����
����H2(g)+I2(g)![]() 2HI(g) ��H=QkJ��mol-1��Ӧ�У�����Ӧ����Ϊv��=k����c(H2)��c(I2)���淴Ӧ����Ϊv��=k����c2(HI)������k����k��Ϊ���ʳ��������¶�Ϊ716Kʱ��
2HI(g) ��H=QkJ��mol-1��Ӧ�У�����Ӧ����Ϊv��=k����c(H2)��c(I2)���淴Ӧ����Ϊv��=k����c2(HI)������k����k��Ϊ���ʳ��������¶�Ϊ716Kʱ��![]() =___���г�����ʽ����
=___���г�����ʽ����
��H2(g)+I2(g)![]() 2HI(g)��ƽ������¶ȣ�ƽ����������ƶ���ԭ����___��
2HI(g)��ƽ������¶ȣ�ƽ����������ƶ���ԭ����___��
��3��һ�������£�NaClO�ɽ���Һ�е�I-����ΪI2��ͨ���ⶨ��ϵ������ȣ�����ͬpH��I2����������ʱ��ı仯��ϵ��ͼ��

��֪�������Խ�߱�������ϵ��c(I2)Խ��
��pH=4.8ʱ����___min����c(I2)���
��10minʱ����ͬpH������ȵĹ�ϵ��___��
��pH=4.0ʱ����ϵ������Ⱥܿ�ﵽ���ֵ��֮������½�������ȿ����½��Ŀ���ԭ����___��