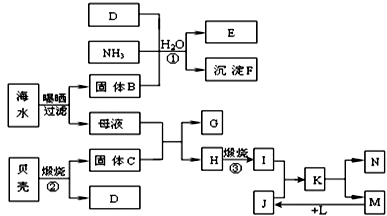
��Ŀ����
���³�ѹ��,��a mol CO2����ͨ��1 L b mol/L��NaOH��Һ��,���ж�������Һ����������ȷ���ǣ� ��
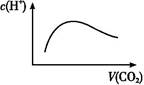

| A����a=2bʱ,����CO2�����ͨ��,��Һ����ˮ�������c(H+)����ͼ�仯��ϵ |
B����a=bʱ,������Һ�д���:c(OH-)+c(C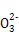 )=c(H+)+c(H2CO3) )=c(H+)+c(H2CO3) |
C����2a=bʱ,������Һ�д���:c(Na+)��c(C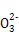 )��c(OH-)��c(HC )��c(OH-)��c(HC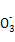 )��c(H+) )��c(H+) |
D����1/2��a/b��1ʱ,������Һ��һ������:c(Na+)=c(C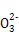 )+c(HC )+c(HC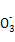 )+c(H2CO3) )+c(H2CO3) |
D
��a=2bʱ,��CO2��ͨ��CO2��NaOH��Ӧ������Na2CO3,�̶�����NaHCO3,��������CO2��H2CO3��ʽ��������Һ��,�����ε�ˮ��ٽ�ˮ�ĵ���,����������ˮ�ĵ���,A��;��a=bʱ,CO2��NaOH��Ӧ����NaHCO3,���������غ��֪c(OH-)=c(H2CO3)+[c(H+)-c(C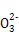 )],B��;��2a=bʱ,CO2��NaOH��Ӧ����Na2CO3,c(OH-)��C
)],B��;��2a=bʱ,CO2��NaOH��Ӧ����Na2CO3,c(OH-)��C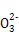 ˮ���ˮ�ĵ����������ṩ,��c(OH-)��c(HC
ˮ���ˮ�ĵ����������ṩ,��c(OH-)��c(HC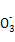 ),C��;��1/2��a/b��1ʱ,CO2��NaOH��Ӧ����Na2CO3��NaHCO3,���������غ�,c(Na+)��c(C
),C��;��1/2��a/b��1ʱ,CO2��NaOH��Ӧ����Na2CO3��NaHCO3,���������غ�,c(Na+)��c(C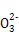 )+c(HC
)+c(HC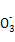 )+c(H2CO3)��
)+c(H2CO3)�� c(Na+),D����
c(Na+),D����
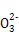 )],B��;��2a=bʱ,CO2��NaOH��Ӧ����Na2CO3,c(OH-)��C
)],B��;��2a=bʱ,CO2��NaOH��Ӧ����Na2CO3,c(OH-)��C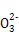 ˮ���ˮ�ĵ����������ṩ,��c(OH-)��c(HC
ˮ���ˮ�ĵ����������ṩ,��c(OH-)��c(HC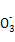 ),C��;��1/2��a/b��1ʱ,CO2��NaOH��Ӧ����Na2CO3��NaHCO3,���������غ�,c(Na+)��c(C
),C��;��1/2��a/b��1ʱ,CO2��NaOH��Ӧ����Na2CO3��NaHCO3,���������غ�,c(Na+)��c(C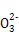 )+c(HC
)+c(HC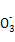 )+c(H2CO3)��
)+c(H2CO3)�� c(Na+),D����
c(Na+),D����
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
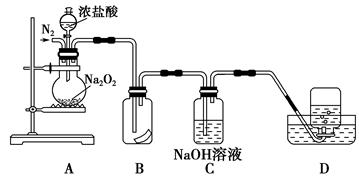
 Na2O2��
Na2O2��