��Ŀ����
18�����������ж���������ѻ�������õĺ����ݲ˳����������Σ��������οɽ�������Ѫ�쵰�������ɸ���Ѫ�쵰�ף�Ѫ�쵰���е���Ԫ���ɶ��۱�Ϊ���ۣ�ʧȥЯ��������ʹ��֯����ȱ����������ˮ���߲˸�����ά����C�����������ж�����Ч�ⶾ��������˵���У�����ȷ���ǣ�������| A�� | ���ж�������Ѫ�쵰�ױ����� | B�� | �ⶾʱѪ�쵰�ױ���ԭ | ||
| C�� | ά����CӦ���л�ԭ�� | D�� | �ж�ʱ�������η���������Ӧ |
���� �������ƽ�������ɽ�ѪҺ�еĵ���Ѫ�쵰�������ɸ���Ѫ�쵰�ף����������ξ��������ԣ�FeԪ�صĻ��ϼ����ߣ�ʹ�����ж���ά����C�����������ж�����Ч�ⶾ������ά����C���л�ԭ�ԣ��������ӷ�Ӧ���ɽⶾ���Դ������
��� �⣺A�����ж�������Ѫ�쵰����FeԪ�صĻ��ϼ����ߣ�����������A��ȷ��
B���ⶾʱѪ�쵰����FeԪ�صĻ��ϼ۽��ͣ�����ԭ����B��ȷ��
C��ά����C�ڽⶾ�����У������仹ԭ�ԣ�����ԭ������C��ȷ��
D�����ж�������Ѫ�쵰����FeԪ�صĻ��ϼ����ߣ����������������εĻ��ϼ۽��ͱ���ԭ��������ԭ��Ӧ����D����
��ѡD��
���� ���⿼����������ԭ��Ӧ������������ԭ�����жϣ���ȷ���������ں��ǽⱾ��ؼ�������Ԫ�ػ��ϼ��Ƿ�仯����������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
8�������й��ڳ����ܽ�ƽ��˵����ȷ���ǣ�������
| A�� | �����β����ڳ����ܽ�ƽ�� | |
| B�� | ��ͨ��������ҺpH����������ʹijЩ�������ӳ��� | |
| C�� | ij��������Һ�г�����ȫ�������������Һ���Ѳ����� | |
| D�� | һ�㣬�ܽ��С����ת�����ܽ�ȸ�С�ģ����ܽ�ȸ�С�IJ���ת�����ܽ��С�� |
9���������ʣ����侧����۵��ɸߵ���˳��������ȷ���ǣ�������
| A�� | SiO2�� CsCl����CBr4��CF4 | B�� | SiO2�� CsCl CF4 ��CBr4 | ||
| C�� | CsCl����SiO2�� CBr4�� CF4 | D�� | CF4 ��CBr4 CsCl����SiO2 |
6�����и��鷴Ӧ�У�����ȱ����������ֱ������Ե��ǣ�������
| A�� | ����ͭ��ϡ���ᷴӦ | B�� | ͭ��ϡ���ᷴӦ | ||
| C�� | ľ̿��Ũ���ᷴӦ | D�� | ̼��Ƹ�ϡ���ᷴӦ |
13������˵������ȷ���ǣ�������
| A�� | �����£��������������Ƶ�������ʢװŨ�����Ũ���� | |
| B�� | ʵ�����У�ʢװNaOH��Һ���Լ�ƿ����Ƥ�� | |
| C�� | ʵ�����У���������Ͳ���ζ��ܡ�����ƿȷ��ȡһ�������Һ�����ڻ�ѧ��Ӧ�� | |
| D�� | ʵ�����У�ʢ���������ò���ƿ |
7��25�棬��c��CH3COOH��+c��CH3COO-��=0.1mol•L-1��һ�����ʹ����ƻ����Һ����Һ��c��CH3COOH����c��CH3COO-����pHֵ�Ĺ�ϵ��ͼ��ʾ�������й�����Ũ�ȹ�ϵ������ȷ���ǣ�������

| A�� | pH=5.5��Һ�У�c��CH3COOH����c��CH3COO-����c��H+����c��OH-�� | |
| B�� | W���ʾ��Һ�У�c��Na+���Tc��CH3COO-��+c��CH3COOH�� | |
| C�� | pH=3.5��Һ�У�c��Na+��+c��H+��-c��OH-��+c��CH3COOH��=0.1mol•L-1 | |
| D�� | ��W������ʾ��1.0L��Һ��ͨ��0.05molHCl���壨��Һ����仯�ɺ��ԣ���c��H+���Tc��CH3COOH��+c��OH-�� |
8����֪��2KMnO4+16HCl=2MnCl2+2KCl+5Cl2��+8H2O��������˵������ȷ���ǣ�������
| A�� | ��ԭ����HCl����������KMnO4 | |
| B�� | ÿ����1mol Cl2ת�Ƶ��ӵ����ʵ���Ϊ2mol | |
| C�� | �������뻹ԭ�������ʵ���֮��Ϊ1��8 | |
| D�� | ���������뻹ԭ��������ʵ���֮��Ϊ5��2 |
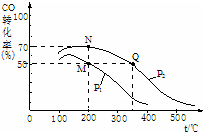 ������Ⱦ������������ȫ���������ŵĿ��⣮��ش��������⣺
������Ⱦ������������ȫ���������ŵĿ��⣮��ش��������⣺