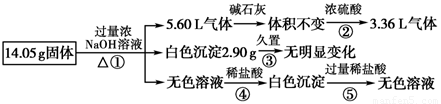
��Ŀ����
ij�������������Al��(NH4)2SO4��MgCl2��AlCl3��FeCl2�е�һ�ֻ�����ɣ��ֶԸû����������ʵ�飬����������й�������ͼ��ʾ(������������ѻ���ɱ�״���µ����)��
�ش��������⣺
��1����������Ƿ����FeCl2 ___(��ǡ���)��
��2����������Ƿ����(NH4)2SO4 ___(��ǡ���)������ж������� ��
��3��д����Ӧ�ܵ����ӷ�Ӧʽ�� __ ��
��4������ݼ������жϻ�������Ƿ���AlCl3(˵����ļ������ݣ���Ҫ��д�������)
��
��5����AlCl3��Һ�м���Ũ�����ɣ����ܵõ�AlCl3��6H2O���壬�������һ�����еļ���ʵ�鷽������������Һ�еõ��ϴ���AlCl3��6H2O���塣
��
��1����2���ǣ� ����ͨ��Ũ�������4.48L��
��3�� AlO2����H����H2O ==Al(OH)3����
��4����������Ϣ���Ƶ�һ������Al��(NH4)2SO4��MgCl2�������ʣ�����������������ʵ�����֮�պõ���28.1g������һ��û��AlCl3��
��5�����Ȼ�����Һ�м���һ������Ũ���ᣬ����Ũ�������½ᾧ�����ˡ�
����:


����˵����ȷ���ǣ�������
| A������������һ������Al������������ȷ�� | B�����������п��ܺ���MgCl2��AlCl3 | C������������һ������MgCl2��FeCl2 | D������������һ�����У�NH4��2SO4��MgCl2 |