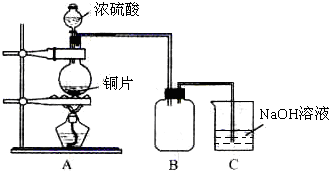
��Ŀ����
��18mol/L����������100mL 1.0mol/L�����ᣬ��ʵ�������У�A��100mL��Ͳ��B��������ƽ��C����������D��50mL����ƿ��E��10mL��Ͳ��F����ͷ�ιܣ�G��50mL�ձ���H��100mL����ƿ��
��1��ʵ��ʱѡ���������Ⱥ�˳����
��2��������ƿ��ʹ�÷����У����в�������ȷ����
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô�����Һ��ϴ
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵĵ�������ƿ�У���������ˮ���ӽ��̶���2-3cm�������õιܵμ�����ˮ���̶���
D��������Һʱ�����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������������ˮ���ӽ��̶���2-3cm�������õιܵμ�����ˮ���̶���
E���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ�����Σ�
��1��ʵ��ʱѡ���������Ⱥ�˳����
F��E��G��C��H
F��E��G��C��H
����2��������ƿ��ʹ�÷����У����в�������ȷ����
B��C��D
B��C��D
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô�����Һ��ϴ
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵĵ�������ƿ�У���������ˮ���ӽ��̶���2-3cm�������õιܵμ�����ˮ���̶���
D��������Һʱ�����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������������ˮ���ӽ��̶���2-3cm�������õιܵμ�����ˮ���̶���
E���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ�����Σ�
��������1������ʵ������IJ����Լ�ÿ��������Ҫ����ȷ����Ӧ����������
��2������ƿֻ���ڳ�����ʹ�ã���������ϡ����Һ����ʹ��ǰҪ����Ƿ�©ˮ�������ô�����Һ��ϴ��
��2������ƿֻ���ڳ�����ʹ�ã���������ϡ����Һ����ʹ��ǰҪ����Ƿ�©ˮ�������ô�����Һ��ϴ��
����⣺��1����Ӧ������������ȡ���ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����
������10ml��Ͳ��ȡ
=5.6mlŨ���ᣬע��ԼΪ5.6mlʱ�����ý�ͷ�ιܵμӣ����ձ����ܽ⣬��ȴ��ת�Ƶ�500ml����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�
�ʴ�Ϊ��F��E��G��C��H��
��2��A������ƿ��ʹ��ǰҪ����Ƿ�©ˮ�������ƺ�Ҫҡ�ȣ���A��ȷ��
B������ƿ������ˮϴ�������ô�����Һ��ϴ���ᵼ����ҺŨ��ƫ��B����
C������ƿֻ���ڳ�����ʹ�ã���������ϡ����Һ����B����
D��Ũ������������Һʱ��������ˮ���ȣ����Ա��������ձ���ϡ�ͣ����ָ�����ʱ������ת�ƽ�����ƿ����C����
E���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ�����Σ�Ŀ����ʹ��Һ��ֻ�ϣ�Ũ�Ⱦ��ȣ���E��ȷ��
�ʴ�Ϊ��BCD����
������10ml��Ͳ��ȡ
| 0.1L��1.0mol/L |
| 18mol/L |
�ʴ�Ϊ��F��E��G��C��H��
��2��A������ƿ��ʹ��ǰҪ����Ƿ�©ˮ�������ƺ�Ҫҡ�ȣ���A��ȷ��
B������ƿ������ˮϴ�������ô�����Һ��ϴ���ᵼ����ҺŨ��ƫ��B����
C������ƿֻ���ڳ�����ʹ�ã���������ϡ����Һ����B����
D��Ũ������������Һʱ��������ˮ���ȣ����Ա��������ձ���ϡ�ͣ����ָ�����ʱ������ת�ƽ�����ƿ����C����
E���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ�����Σ�Ŀ����ʹ��Һ��ֻ�ϣ�Ũ�Ⱦ��ȣ���E��ȷ��
�ʴ�Ϊ��BCD����
���������⿼������һ�����ʵ���Ũ����Һ��ʵ���������Ŀ�ѶȲ�����ע����Ͳ��ѡ��

��ϰ��ϵ�д�
 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
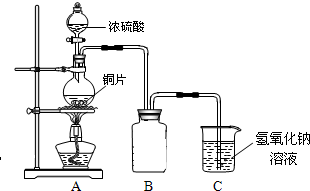 ��2011?����ģ�⣩ij��ѧ��ȤС��Ϊ̽��
��2011?����ģ�⣩ij��ѧ��ȤС��Ϊ̽��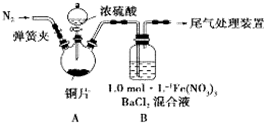 ��ʡ��2013��12��1����ʱ�𣬳�����������Ϊ�����������������е������½�����֮�����Զ���������ŷ����˴��ĸ��ƣ�SO2������Fe�� NO3��3��Һ���գ�ij��ѧ��ȤС���SO2�� Fe�� NO3��3 ��Һ�ķ�Ӧ[0.1mol/L��Fe��NO3��3 ��Һ�� pH=2]������Ӧ̽����
��ʡ��2013��12��1����ʱ�𣬳�����������Ϊ�����������������е������½�����֮�����Զ���������ŷ����˴��ĸ��ƣ�SO2������Fe�� NO3��3��Һ���գ�ij��ѧ��ȤС���SO2�� Fe�� NO3��3 ��Һ�ķ�Ӧ[0.1mol/L��Fe��NO3��3 ��Һ�� pH=2]������Ӧ̽����