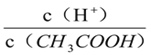ƒøƒ⁄»ð
°æƒø°ø ؔա—Ω‚∆¯÷˜“™∫¨”–±˚œ©°¢1,3-∂°∂˛œ©µ»≤ª±•∫ÕÃ˛£¨“‘À¸√«Œ™‘≠¡œø…∫œ≥…CRœΩ∫∫Õ“Ω“©÷–º‰ÃÂG,£¨∫œ≥…¬∑œþ»Áœ¬£∫

“—÷™£∫¢ŸB°¢C°¢D æ˘ƒÐ∑¢…˙“¯æµ∑¥”¶£ª
¢⁄
£®1£©AµƒÀ≥ Ω“ÏππõƒΩ·ππºÚ ΩŒ™___________________°£
£®2£©C÷–∫¨—ıπŸƒÐÕ≈µƒ√˚≥∆ «____________£¨∑¥”¶¢Ÿµƒ∑¥”¶¿ý–ÕŒ™____________________°£
£®3£©–¥≥ˆE°˙ ∑¥”¶µƒªØ—ß∑Ω≥Ã Ω£∫_________________________________°£
£®4£©–¥≥ˆÕ¨ ±¬˙◊„œ¬¡–Ãıº˛µƒ“Ω“©÷–º‰ÃÂGµƒÕ¨∑÷“ÏππõƒΩ·ππºÚ Ω£∫ __________________°£
¢Ÿ”ÎD ª•Œ™Õ¨œµŒÔ£ª ¢⁄∫À¥≈π≤’Ò«‚∆◊”–»˝◊È∑°£
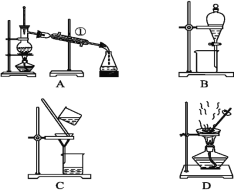
£®5£©”√ºÚ“™”Ô—‘±Ì ˆºÏ—ÈB÷–À˘∫¨πŸƒÐÕ≈µƒ µ—È∑Ω∑®£∫_______________________°£
£®6£©“‘AŒ™∆ º‘≠¡œ∫œ≥…CRœΩ∫µƒœþ¬∑Œ™______________________£®∆‰À¸ ‘º¡»Œ—°£©°£
°æ¥∞∏°ø 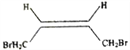 Ù«ª˘°¢»©ª˘ »°¥˙∑¥”¶ HOOCCH2COOH+2C2H5OH
Ù«ª˘°¢»©ª˘ »°¥˙∑¥”¶ HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O OHC(CH2)4CHO°¢OHCCH(CH3)CH(CH3)CHO »°…Ÿ¡øB”⁄Ωýæª ‘πÐ÷–£¨º”»Î◊„¡ø“¯∞±»Ð“∫£¨ÀÆ‘°º”»»”–“¯æµ…˙≥…£¨÷§√˜B÷–”–»©ª˘£ª‘Ÿº”À·À·ªØ£¨µŒ»Î…Ÿ¡ø‰ÂµƒÀƒ¬»ªØú»Ð“∫£¨»Ð“∫Õ …´£¨÷§√˜∫¨”–úúÀ´º¸
C2H5OOCCH2COOC2H5+2H2O OHC(CH2)4CHO°¢OHCCH(CH3)CH(CH3)CHO »°…Ÿ¡øB”⁄Ωýæª ‘πÐ÷–£¨º”»Î◊„¡ø“¯∞±»Ð“∫£¨ÀÆ‘°º”»»”–“¯æµ…˙≥…£¨÷§√˜B÷–”–»©ª˘£ª‘Ÿº”À·À·ªØ£¨µŒ»Î…Ÿ¡ø‰ÂµƒÀƒ¬»ªØú»Ð“∫£¨»Ð“∫Õ …´£¨÷§√˜∫¨”–úúÀ´º¸ 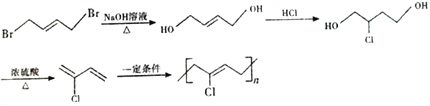
°æΩ‚Œˆ°ø∏˘æð¡˜≥ÃÕº£¨1,3-∂°∂˛œ©”ΉÂ∑¢…˙1,4º”≥…£¨…˙≥…A£¨AŒ™1,4-∂˛‰Â-2-∂°œ©(![]() )£ª”Ϋ‚∆¯º”≥…∫Û…˙≥…1,4-∂˛‰Â∂°ÕÈ(
)£ª”Ϋ‚∆¯º”≥…∫Û…˙≥…1,4-∂˛‰Â∂°ÕÈ(![]() )£ª±˚œ©¥þªØ—ıªØ…˙≥…B£¨BŒ™CH2=CHCHO£¨Cµƒœý∂‘∑÷◊”÷ ¡ø±»B¥Û18£¨Àµ√˜B”ÎÀƺ”≥……˙≥…C£¨CŒ™
)£ª±˚œ©¥þªØ—ıªØ…˙≥…B£¨BŒ™CH2=CHCHO£¨Cµƒœý∂‘∑÷◊”÷ ¡ø±»B¥Û18£¨Àµ√˜B”ÎÀƺ”≥……˙≥…C£¨CŒ™![]() £¨¥þªØ—ıªØ…˙≥…D(±˚∂˛»©)£¨”Γ¯∞±»Ð“∫∑¥”¶…˙≥…E(±˚∂˛À·)£¨”Γ“¥ºı•ªØ∑¥”¶…˙≥…F(
£¨¥þªØ—ıªØ…˙≥…D(±˚∂˛»©)£¨”Γ¯∞±»Ð“∫∑¥”¶…˙≥…E(±˚∂˛À·)£¨”Γ“¥ºı•ªØ∑¥”¶…˙≥…F(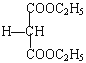 )£ª∏˘æð–≈œ¢£¨F(
)£ª∏˘æð–≈œ¢£¨F( )”Î
)”Î![]() ∑¥”¶…˙≥…
∑¥”¶…˙≥…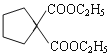 £¨Ω¯“ª≤Ω∑¥”¶…˙≥…G(
£¨Ω¯“ª≤Ω∑¥”¶…˙≥…G(![]() )°£
)°£
(1)AŒ™1,4-∂˛‰Â-2-∂°œ©(![]() )£¨∆‰À≥ Ω“ÏππõƒΩ·ππºÚ ΩŒ™
)£¨∆‰À≥ Ω“ÏππõƒΩ·ππºÚ ΩŒ™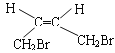 £¨’˝»∑¥∞∏£∫
£¨’˝»∑¥∞∏£∫ °£
°£
(2) CŒ™![]() £¨∫¨”–µƒπŸƒÐÕ≈”–úú٫ª˘°¢»©ª˘£ªÕ®π˝Ã‚∏¯–≈œ¢¢⁄ø…÷™£¨∏√π˝≥Ã∑¢…˙¡À»°¥˙∑¥”¶£ª’˝»∑¥∞∏£∫úúÀ´º¸°¢»©ª˘£ª »°¥˙∑¥”¶°£
£¨∫¨”–µƒπŸƒÐÕ≈”–úú٫ª˘°¢»©ª˘£ªÕ®π˝Ã‚∏¯–≈œ¢¢⁄ø…÷™£¨∏√π˝≥Ã∑¢…˙¡À»°¥˙∑¥”¶£ª’˝»∑¥∞∏£∫úúÀ´º¸°¢»©ª˘£ª »°¥˙∑¥”¶°£
£®3£©”–ª˙ŒÔEŒ™HOOCCH2COOH£¨”ÎC2H5OH‘⁄≈®¡ÚÀ·º”»»µƒÃıº˛œ¬∑¢…˙ı•ªØ∑¥”¶£¨ªØ—ß∑Ω≥Ã Ω£∫HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O £ª’˝»∑¥∞∏£∫HOOCCH2COOH+2C2H5OH
C2H5OOCCH2COOC2H5+2H2O £ª’˝»∑¥∞∏£∫HOOCCH2COOH+2C2H5OH![]() C2H5OOCCH2COOC2H5+2H2O°£
C2H5OOCCH2COOC2H5+2H2O°£
£®4£©”–ª˙ŒÔGµƒ∑÷◊” ΩŒ™C6H10O2, ¢Ÿ”ÎD ª•Œ™Õ¨œµŒÔ£ª”–ª˙ŒÔDŒ™∂˛‘™»©¿ý£ª¢⁄∫À¥≈π≤’Ò«‚∆◊”–»˝◊È∑£¨æþ”–“ª∂®µƒ∂‘≥∆–‘£ª¬˙◊„Ãıº˛µƒ”–ª˙ŒÔø…ƒÐµƒΩ·ππ”–2÷÷£∫OHC(CH2)4CHO∫ÕOHCCH(CH3)CH(CH3)CHO£ª’˝»∑¥∞∏£∫ OHC(CH2)4CHO∫ÕOHCCH(CH3)CH(CH3)CHO°£
£®5£©”–ª˙ŒÔB CH2=CHCHO£¨∫¨”–»©ª˘∫ÕúúÀ´º¸£¨”…”⁄»©ª˘µƒªπ‘≠–‘Ωœ«ø£¨œ»ºÏ—È»©ª˘£¨»ª∫Û‘⁄ºÏ—ÈúúÀ´º¸£ªæþÃÂ≤Ÿ◊˜»Áœ¬£∫»°…Ÿ¡øB”⁄Ωýæª ‘πÐ÷–£¨º”»Î◊„¡ø“¯∞±»Ð“∫£¨ÀÆ‘°º”»»”–“¯æµ…˙≥…£¨÷§√˜B÷–”–»©ª˘£ª‘Ÿº”À·À·ªØ£¨µŒ»Î…Ÿ¡ø‰ÂµƒÀƒ¬»ªØú»Ð“∫£¨»Ð“∫Õ …´£¨÷§√˜∫¨”–úúÀ´º¸ £ª’˝»∑¥∞∏£∫»°…Ÿ¡øB”⁄Ωýæª ‘πÐ÷–£¨º”»Î◊„¡ø“¯∞±»Ð“∫£¨ÀÆ‘°º”»»”–“¯æµ…˙≥…£¨÷§√˜B÷–”–»©ª˘£ª‘Ÿº”À·À·ªØ£¨µŒ»Î…Ÿ¡ø‰ÂµƒÀƒ¬»ªØú»Ð“∫£¨»Ð“∫Õ …´£¨÷§√˜∫¨”–úúÀ´º¸ °£
£®6£©AŒ™![]() £¨‘⁄ºÓ–‘ª∑æ≥œ¬∑¢…˙»°¥˙∑¥”¶…˙≥…∂˛‘™œ©¥º
£¨‘⁄ºÓ–‘ª∑æ≥œ¬∑¢…˙»°¥˙∑¥”¶…˙≥…∂˛‘™œ©¥º![]() £¨»ª∫Û‘Ÿ”ά»ªØ«‚∑¢…˙º”≥……˙≥…
£¨»ª∫Û‘Ÿ”ά»ªØ«‚∑¢…˙º”≥……˙≥…![]() £¨∏√”–ª˙ŒÔ‘⁄≈®¡ÚÀ·◊˜”√œ¬∑¢…˙œ˚»•∑¥”¶…˙≥…
£¨∏√”–ª˙ŒÔ‘⁄≈®¡ÚÀ·◊˜”√œ¬∑¢…˙œ˚»•∑¥”¶…˙≥…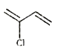 £ª∏√”–ª˙ŒÔ∑¢…˙º”æ€∑¥”¶…˙≥…∏þ∑÷◊”£ªæþá˜≥ûÁœ¬£∫
£ª∏√”–ª˙ŒÔ∑¢…˙º”æ€∑¥”¶…˙≥…∏þ∑÷◊”£ªæþá˜≥ûÁœ¬£∫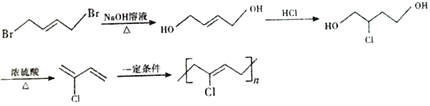 £ª’˝»∑¥∞∏£∫
£ª’˝»∑¥∞∏£∫ °£
°£

 ‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
‘ƒ∂¡øÏ≥µœµ¡–¥∞∏°æƒø°ø𧓵…œ”√∫¨»˝º€∑∞£®V2O3£©Œ™÷˜µƒƒ≥ Ø√∫Œ™‘≠¡œ£®∫¨”–Al2O3°¢CaOµ»‘”÷ £©£¨∏∆ªØ∑®±∫…’÷∆±∏V2O5£¨∆‰¡˜≥ûÁœ¬£∫
![]()
°æ◊ ¡œ°ø£∫+5º€∑∞‘⁄»Ð“∫÷–µƒ÷˜“™¥Ê‘⁄–Œ Ω”Î»Ð“∫pHµƒπÿœµ£∫
pH | 4~6 | 6~8 | 8~10 | 10~12 |
÷˜“™¿Î◊” | VO2+ | VO3 | V2O74 | VO43 |
£®1£©±∫…’£∫œÚ Ø√∫÷–º”…˙ ت“±∫…’£¨Ω´V2O3◊™ªØŒ™Ca(VO3)2µƒªØ—ß∑Ω≥Ã Ω «______°£
£®2£©À·Ω˛£∫ ¢Ÿ Ca(VO3)2ƒ—»Ð”⁄ÀÆ£¨ø…»Ð”⁄—ŒÀ·°£»Ù±∫…∞À·Ω˛ ±»Ð“∫µƒpH£Ω4£¨Ca(VO3)2»Ð”⁄—ŒÀ·µƒ¿Î◊”∑Ω≥Ã Ω «______°£
¢⁄ À·∂»∂‘∑∞∫Õ¬¡µƒ»ÐΩ‚¡øµƒ”∞œÏ»ÁÕºÀ˘ æ£∫À·Ω˛ ±»Ð“∫µƒÀ·∂»øÿ÷∆‘⁄¥Û‘º3.2%£¨∏˘æð»ÁÕºÕ∆≤‚£¨À·Ω˛ ±≤ª—°‘Ò∏¸∏þÀ·∂»µƒ‘≠“Ú «______°£

£®3£©◊™≥¡£∫Ω´Ω˛≥ˆ“∫÷–µƒ∑∞◊™ªØŒ™NH4VO3πÃã¨∆‰¡˜≥ûÁœ¬£∫
![]()
¢Ÿ Ω˛≥ˆ“∫÷–º”»Î ت“»Èµƒ◊˜”√ «______°£
¢⁄ “—÷™CaCO3µƒ»ÐΩ‚∂»–°”⁄Ca3(VO4)2°£œÚCa3(VO4)2≥¡µÌ÷–º”»Î(NH4)2CO3»Ð“∫£¨ø… π∑∞¥”≥¡µÌ÷–»Ð≥ˆ°£Ω·∫œªØ—ß”√”Ô£¨”√∆Ω∫‚“∆∂Ø‘≠¿ÌΩ‚ Õ∆‰‘≠“Ú£∫______°£
¢€ œÚ(NH4)3VO4»Ð“∫÷–º”»ÎNH4Cl»Ð“∫£¨øÿ÷∆»Ð“∫µƒpH£Ω7.5°£µ±pH£æ8 ±£¨NH4VO3µƒ≤˙¡ø√˜œ‘ΩµµÕ£¨‘≠“Ú «______°£
£®4£©≤‚∂®≤˙∆∑÷–V2O5µƒ¥ø∂»£∫
≥∆»°a g≤˙∆∑£¨œ»”√¡ÚÀ·»ÐΩ‚£¨µ√µΩ(VO2)2SO4»Ð“∫°£‘Ÿº”»Îb1 mL c1 mol°§L1 (NH4)2Fe(SO4)2»Ð“∫£®VO2+ + 2H+ + Fe2+ == VO2+ + Fe3+ + H2O£©°£◊Ó∫Û”√c2 mol°§L1 KMnO4»Ð“∫µŒ∂®π˝¡øµƒ(NH4)2Fe(SO4)2÷¡÷’µ„£¨œ˚∫ƒKMnO4»Ð“∫µƒÃª˝Œ™b2 mL°£“—÷™ MnO4±ªªπ‘≠Œ™Mn2+£¨ºŸ…Ë‘”÷ ≤ª≤Œ”Î∑¥”¶°£‘Ú≤˙∆∑÷–V2O5µƒ÷ ¡ø∑÷ ˝ «______°££®V2O5µƒƒ¶∂˚÷ ¡ø£∫182 g°§mol1£©