��Ŀ����
(8��)��֪��ϩ��R��2CH==1CH2��HBr�ӳɷ�Ӧ�У�Brԭ�Ӳ���ɼ��ķ�ʽ�����ֿ��ܣ���Brԭ����1C�ϳɼ�����Brԭ����2C�ϳɼ�����ѧ��ȤС������0.224 L��ϩ(��״̬)������HBr���мӳɷ�Ӧ��ϣ��ͨ������ʵ��ķ���̽����ӳɷ�ʽ��ʵ�鲽�����£�
(һ)��ϩ��HBr���ʵ������¼ӳɵõ�±������
(��)±������NaOHˮ��Һ�м��ȣ�
(��)�ᴿ�õ���ϴ���
(��)����ϴ�����ͨ������������ͭ����
(��)�������ͨ��������������Һ�У�����������Ӧʵ�飬��ȫ��Ӧ�����յõ�W������
����������⣺
(1)����(��)���ᴿ��ϴ��ļ����������ǣ�������ϴ��Ļ�������__________��
(2)ʵ������β�����������������
(3)д������(��)�е�����һ����ѧ��Ӧ����ʽ��(ֻдһ��)
(4)С���ԱԤ�ڿ���ʵ���������ۣ�(��ʾ������Ӧ�����ձ��ԣ��ο���ʽ�硰ϩ����HBr�ӳɵķ�ʽΪ��/�ڡ�)
(һ)��ϩ��HBr���ʵ������¼ӳɵõ�±������
(��)±������NaOHˮ��Һ�м��ȣ�
(��)�ᴿ�õ���ϴ���
(��)����ϴ�����ͨ������������ͭ����
(��)�������ͨ��������������Һ�У�����������Ӧʵ�飬��ȫ��Ӧ�����յõ�W������
����������⣺
(1)����(��)���ᴿ��ϴ��ļ����������ǣ�������ϴ��Ļ�������__________��
(2)ʵ������β�����������������
(3)д������(��)�е�����һ����ѧ��Ӧ����ʽ��(ֻдһ��)
(4)С���ԱԤ�ڿ���ʵ���������ۣ�(��ʾ������Ӧ�����ձ��ԣ��ο���ʽ�硰ϩ����HBr�ӳɵķ�ʽΪ��/�ڡ�)
(1)����
(2)��ȡһ֧����ྻ���Թܵ�����m1���ô��Թܽ�������ʵ�飬ʵ����ϣ������Թ��е���Һ��������ˮϴ���Թܼ��Σ�Ȼ���ɣ��ٳ����Թ���ͬ��������m2,(m2-m1)��Ϊ��������w��
(3)2CH3CH2CH2OH+O2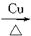 2CH3CH2CHO+2H2O(��
2CH3CH2CHO+2H2O(��
 +2H2O)
+2H2O)
(4)��wΪ1.08,��ϩ����HBr�ļӳɷ�ʽΪ��
��w=0����ϩ����HBr�ļӳɷ�ʽΪ��
��0<w<1.08,��ϩ����HBr�ӳɵķ�ʽ�٢ھ���
(2)��ȡһ֧����ྻ���Թܵ�����m1���ô��Թܽ�������ʵ�飬ʵ����ϣ������Թ��е���Һ��������ˮϴ���Թܼ��Σ�Ȼ���ɣ��ٳ����Թ���ͬ��������m2,(m2-m1)��Ϊ��������w��
(3)2CH3CH2CH2OH+O2
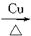 2CH3CH2CHO+2H2O(��
2CH3CH2CHO+2H2O(�� +2H2O)
+2H2O)(4)��wΪ1.08,��ϩ����HBr�ļӳɷ�ʽΪ��
��w=0����ϩ����HBr�ļӳɷ�ʽΪ��
��0<w<1.08,��ϩ����HBr�ӳɵķ�ʽ�٢ھ���
(2)Ϊ��֤���ɵ�������ʧ����С�����ܽ������£�Ҳ������ȫ�����¡�
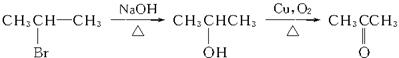
(4)CH3CH==CH2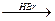 CH3CH2CH2Br
CH3CH2CH2Br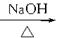 CH3CH2CH2OH
CH3CH2CH2OH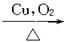 CH3CH2CHO
CH3CH2CHO
CH3CH==CH2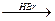
(4)CH3CH==CH2
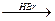 CH3CH2CH2Br
CH3CH2CH2Br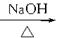 CH3CH2CH2OH
CH3CH2CH2OH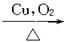 CH3CH2CHO
CH3CH2CHOCH3CH==CH2
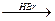


��ϰ��ϵ�д�
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
�����Ŀ
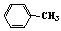 )����b��һ�ȴ����a��b����ֵ�ֱ�Ϊ
)����b��һ�ȴ����a��b����ֵ�ֱ�Ϊ  HBr+NaHSO4 ��
HBr+NaHSO4 �� R-Br+H2O ��
R-Br+H2O �� Ӣ����Ⱦ�������Ӱ���˸ù���ʳƷ������������
Ӣ����Ⱦ�������Ӱ���˸ù���ʳƷ������������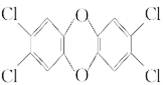 ���йظû������˵������ȷ����( )
���йظû������˵������ȷ����( )