��Ŀ����
��14�֣��������ѧ�ĵ绯ѧԭ��������������⡣
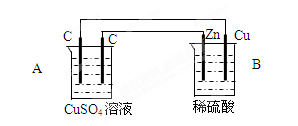
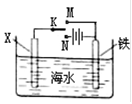
��1������������ԭ��Ӧ��2Ag+(aq) + Cu(s) = Cu2+(aq) + 2Ag(s) ��Ƶ�˫Һԭ��أ������ṩ�ȶ��ĵ�����װ����ͼ��ʾ������������װ����֬������KCl��Һ��

�ش��������⣺
�ٵ缫X�IJ��Ϻ͵������ҺY�ֱ�Ϊ ��
��������K+���� ����A��B����
�����缫�����ĵ缫��ӦΪ ��
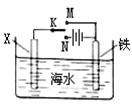
��2����ͼ��ʾһ�����أ�װ�е��Һa ��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
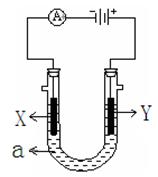
����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ�� ����X��Y���缫�����ȱ��ɫ�� X���ϵĵ缫��ӦʽΪ ��
����Ҫ������Ʒ�϶�һ����ȵ�Cu�㣬Y�缫��ӦʽΪ
����X��Y���Dz��缫�����ij����M���Ȼ��MCl2����Һ�����ռ���1.12 L����ʱ����״��������������3.2 g���ý��������ԭ�������� ��
��1������������ԭ��Ӧ��2Ag+(aq) + Cu(s) = Cu2+(aq) + 2Ag(s) ��Ƶ�˫Һԭ��أ������ṩ�ȶ��ĵ�����װ����ͼ��ʾ������������װ����֬������KCl��Һ��

�ش��������⣺
�ٵ缫X�IJ��Ϻ͵������ҺY�ֱ�Ϊ ��
��������K+���� ����A��B����
�����缫�����ĵ缫��ӦΪ ��
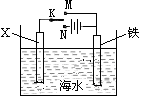
��2����ͼ��ʾһ�����أ�װ�е��Һa ��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺

����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ�� ����X��Y���缫�����ȱ��ɫ�� X���ϵĵ缫��ӦʽΪ ��
����Ҫ������Ʒ�϶�һ����ȵ�Cu�㣬Y�缫��ӦʽΪ
����X��Y���Dz��缫�����ij����M���Ȼ��MCl2����Һ�����ռ���1.12 L����ʱ����״��������������3.2 g���ý��������ԭ�������� ��
�� 14�֣���ÿ��2�֣�
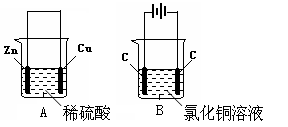
��1����Cu��AgNO3��Һ ��B ��Ag+ + e- = Ag
��2����X 2H2O ��2e-��H2��+OH-(��2H+�� 2e- ��H2�� )
��Cu - 2e- ��Cu2+ ��64
��1����Cu��AgNO3��Һ ��B ��Ag+ + e- = Ag
��2����X 2H2O ��2e-��H2��+OH-(��2H+�� 2e- ��H2�� )
��Cu - 2e- ��Cu2+ ��64
��1������ԭ����нϻ��õĽ�����������ʧȥ���ӣ�����������Ӧ�����Ӿ����ߴ��ݵ������ϣ�������Һ�е��������������ƶ������������ƶ��������õ����ӣ�������ԭ��Ӧ�������ܷ�Ӧʽ��֪��ͭʧȥ���ӣ�����ͭ�Ǹ�������X��ͭ�������ӵõ����ӣ�����������Һ�е��������������Һ��
��ԭ����������������ƶ�����K+����B��
�����缫����������Һ�е������ӵõ����ӣ�����ʽΪAg+ + e- = Ag��
��2���ٶ��Ե缫��ⱥ���Ȼ�����Һ�������������ӷŵ磬�Ӷ��ƻ�������Χˮ�ĵ���ƽ�⣬����������Χ��Һ�Լ��ԣ���Һ�Ժ�ɫ������װ��ͼ��֪��X���Դ�ĸ��������������������Ե缫��ӦʽΪ2H+�� 2e- ��H2�� ��
�ڵ��ʱ�����ƽ������������Ʋ���������������жƲ�������ӵ���Һ���������Һ������װ��ͼ��֪��Y���Դ��������������������������ͭ���缫��ӦʽΪCu - 2e- ��Cu2+��
��1.12 L����ʱ����״������0.05mol��ת�Ƶ�����0.05mol��2��0.1mol�����Ը��ݵ��ӵ�ʧ�غ��֪�����������ԭ��������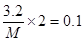 �����M��64��
�����M��64��
��ԭ����������������ƶ�����K+����B��
�����缫����������Һ�е������ӵõ����ӣ�����ʽΪAg+ + e- = Ag��
��2���ٶ��Ե缫��ⱥ���Ȼ�����Һ�������������ӷŵ磬�Ӷ��ƻ�������Χˮ�ĵ���ƽ�⣬����������Χ��Һ�Լ��ԣ���Һ�Ժ�ɫ������װ��ͼ��֪��X���Դ�ĸ��������������������Ե缫��ӦʽΪ2H+�� 2e- ��H2�� ��
�ڵ��ʱ�����ƽ������������Ʋ���������������жƲ�������ӵ���Һ���������Һ������װ��ͼ��֪��Y���Դ��������������������������ͭ���缫��ӦʽΪCu - 2e- ��Cu2+��
��1.12 L����ʱ����״������0.05mol��ת�Ƶ�����0.05mol��2��0.1mol�����Ը��ݵ��ӵ�ʧ�غ��֪�����������ԭ��������
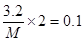 �����M��64��
�����M��64��
��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�
�����Ŀ
 Fe(OH)2 + Ni(OH)2��
Fe(OH)2 + Ni(OH)2��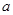 g M�������M�����ԭ������Ϊ ���ú���
g M�������M�����ԭ������Ϊ ���ú���