ЬтФПФкШн
ЧтфхЫсдквНвЉКЭЪЏЛЏЙЄвЕЩЯгаЙуЗКгУЭОЁЃЯТЭМЪЧФЃФтЙЄвЕжЦБИЧтфхЫсДжВњЦЗВЂОЋжЦЕФСїГЬЃК

ИљОнЩЯЪіСїГЬЛиД№ЯТСаЮЪЬтЃК
(1)ЛьКЯЂйжаЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ________________________________ЁЃ
(2)ЛьКЯЂйжаЪЙгУБљЫЎЕФФПЕФЪЧ_______________________________________ЁЃ
(3)ВйзїЂѓвЛАуЪЪгУгкЗжРы________ЛьКЯЮяЁЃ(ЬюађКХ)
aЃЎЙЬЬхКЭвКЬх bЃЎЙЬЬхКЭЙЬЬх
cЃЎЛЅВЛЯрШмЕФвКЬх dЃЎЛЅШмЕФвКЬх
(4)ЛьКЯЂкжаМгШыNa2SO3ЕФФПЕФЪЧ____________________________________ЁЃ
(5)ДПОЛЕФЧтфхЫсгІЮЊЮоЩЋвКЬхЃЌЕЋЪЕМЪЙЄвЕЩњВњжажЦЕУЕФЧтфхЫс(ЙЄвЕЧтфхЫс)ДјгаЕЕЕФЛЦЩЋЁЃгкЪЧМзввСНЭЌбЇЩшМЦСЫМђЕЅЪЕбщМгвдЬНОПЃК
МзЭЌбЇМйЩшЙЄвЕЧтфхЫсГЪЕЛЦЩЋЪЧвђЮЊЦфжаКЌгаFe3ЃЋЃЌдђгУгкжЄУїИУМйЩшЫљгУЕФЪдМСЮЊ________ЃЌШєМйЩшГЩСЂПЩЙлВьЕНЕФЯжЯѓЮЊ__________________ЃЛввЭЌбЇМйЩшЙЄвЕЧтфхЫсГЪЕЛЦЩЋЪЧвђЮЊЦфжаКЌга________________ЃЌЦфгУгкжЄУїИУМйЩшЫљгУЕФЪдМСЮЊ____________ЁЃ
(5)KSCNШмвКЁЁШмвКБфГЩКьЩЋЁЁBr2ЁЁCCl4(ЦфЫћКЯРэ
вВПЩ)
ЁОНтЮіЁПЁЁSO2гыBr2дкЫЎжаЗЂЩњбѕЛЏЛЙдЗДгІЃЌЗДгІЗХШШЃЌЮЊЗРжЙфхеєЗЂЃЌгУБљЫЎНЕЮТЁЃВйзїЂёЁЂЂѓОљЪЧеєСѓЃЌВйзїЂђЪЧЙ§ТЫЁЃЛьКЯЂкжаМгШыNa2SO3ЕФФПЕФЪЧГ§ШЅДжВњЦЗжаЮДЗДгІЭъЕФфхЁЃМьбщFe3ЃЋГЃгУKSCNШмвКЃЛЙЄвЕЧтфхЫсжаПЩФмКЌгафхЖјГЪЕЛЦЩЋЃЌПЩгУЫФТШЛЏЬМнЭШЁЖјжЄУїЁЃ

 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИвЛЖЈЮТЖШЯТЃЌдк2 LУмБеШнЦїжаМгШыФЩУзМЖCu2OВЂЭЈШы0.1 mol H2O(g)ЃЌЗЂЩњЗДгІЃК2H2O(g)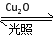 2H2(g)ЃЋO2(g)ЁЁІЄHЃНЃЋ484 kJЁЄmolЃ1ЃЌВЛЭЌЪБМфВњЩњO2ЕФЮяжЪЕФСПМћЯТБэЃК
2H2(g)ЃЋO2(g)ЁЁІЄHЃНЃЋ484 kJЁЄmolЃ1ЃЌВЛЭЌЪБМфВњЩњO2ЕФЮяжЪЕФСПМћЯТБэЃК
ЪБМф/min | 20 | 40 | 60 | 80 |
n(O2)/mol | 0.001 0 | 0.001 6 | 0.002 0 | 0.002 0 |
ЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ (ЁЁЁЁ)ЁЃ
AЃЎЧА20 minФкЕФЦНОљЗДгІЫйТЪv(H2O)ЃН5.0ЁС10Ѓ5 molЁЄLЃ1ЁЄminЃ1
BЃЎДяЕНЦНКтЪБЃЌжСЩйашвЊДгЭтНчЮќЪеФмСП0.968 kJ
CЃЎдіДѓc(H2O)ЃЌПЩвдЬсИпЫЎЕФЗжНтТЪ
DЃЎДпЛЏаЇЙћгыCu2OПХСЃЕФДѓаЁгаЙи



