��Ŀ����
����Ŀ����PbSǦ����(��Ҫ�ɷ�ΪPbS������������)̼������Ǧ��һ����ɫ���գ�����Ҫ�����������£�

�ش��������⣺
��1����ת�����Ǹù��յĹؼ���ת��ʱ������Ӧ�Ļ�ѧ����ʽΪ2PbS��2(NH4)2CO3��O2��2H2O===2PbCO3��2S��4NH3��H2O��
��(NH4)2CO3��������Ӧ��________(����ĸ)��
a����������b������ԭ��c���Ȳ���������Ҳ������ԭ��
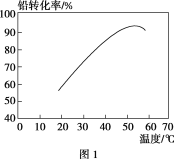
��ת��ʱ���¶ȶ�Ǧת���ʵ�Ӱ����ͼ1��ʾ�������˵�ת���¶ȷ�ΧΪ________��
������FeS2��Ǧת���ʵ�Ӱ����ͼ2��ʾ��˵��FeS2��ת���е�������________��

��2�����ܽ⡱ʱ������Ӧ�����ӷ���ʽΪ________________________________��
��3������⡱ʱ�������ĵ缫��ӦʽΪ___________________________________��
���𰸡�c 50��60 �� ������ PbCO3��2H��===Pb2����CO2����H2O Pb2����2e��===Pb
��������
��1�����������õ����ӣ����ϼ۽��ͣ���ԭ��ʧȥ���ӣ����ϼ����ߣ�
�����˵�ת���¶ȷ�Χ���Ƿ����ĸ���Χ��Ǧת������ߣ�
���ڻ�ѧ��Ӧ���ܸı䷴Ӧ�ﻯѧ��Ӧ���ʣ���ͣ������ı仯ѧƽ�⣬�ұ����������ͻ�ѧ�����ڻ�ѧ��Ӧǰ��û�з����ı�����ʽд�����
��2���ܽ������̼��Ǧ���ᷴӦ����Ǧ�Ρ�������̼��ˮ��
��3�������õ����ӣ����ϼ۽��ͣ�������ԭ��Ӧ��
��1�����������õ����ӣ����ϼ۽��ͣ���ԭ��ʧȥ���ӣ����ϼ����ߣ�(NH4)2CO3�ڷ�Ӧ��Ԫ�صĻ��ϼ�û�䣬��ѡc��
�����˵�ת���¶ȷ�Χ���Ƿ����ĸ���Χ��Ǧת������ߣ����˵�ת���¶ȷ�ΧΪ50��60 ����
���ڻ�ѧ��Ӧ���ܸı䷴Ӧ�ﻯѧ��Ӧ���ʣ���ͣ������ı仯ѧƽ�⣬�ұ����������ͻ�ѧ�����ڻ�ѧ��Ӧǰ��û�з����ı�����ʽд�����FeS2��ת���е���������������
��2���ܽ������̼��Ǧ���ᷴӦ����Ǧ�Ρ�������̼��ˮ�����ܽ⡱ʱ������Ӧ�����ӷ���ʽΪPbCO3��2H��===Pb2����CO2����H2O ��
��3�������õ����ӣ����ϼ۽��ͣ�������ԭ��Ӧ������⡱ʱ�������ĵ缫��ӦʽΪ�� Pb2����2e��===Pb��

����Ŀ��������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���
ѡ�� | �� | �� | �� | ʵ����� |
|
A | ϡ���� | Na2SO3 | Na2SiO3�� | �ǽ����ԣ�S>Si | |
B | Ũ���� | ���� | ��ˮ | Ũ���������ˮ�ԡ������� | |
C | ϡ���� | Na2SO3 | Ba(NO3)2��Һ | SO2������Ա��ξ������ɰ�ɫ���� | |
D | Ũ���� | Na2CO3 | Na2SiO3��Һ | ���ԣ�����>̼��>���� |
A. A B. B C. C D. D
����Ŀ��������ʵ�鼰�������Ƴ���Ӧ���۵��ǣ� ��
ʵ�� | ���� | ���� | |
A | �� | ��Һ��ΪѪ��ɫ |
|
B | �� | ���ɰ�ɫ���� | �ǽ����� |
C | ��ʢ�� | ��ˮ��ɫ | ��ԭ�ԣ� |
D | ����ɫ��Һ�е��� | �а�ɫ�������� | ��ɫ��Һ��һ���� |
A. A B. B C. C D. D
����Ŀ������ʵ���У���Ӧ�������Լ����۶���ȷ�����߾��������ϵ����
ѡ�� | ʵ�� | ���� | ���� |
A | ��ϡ�����м����������ۣ���ַ�Ӧ��μ�KSCN��Һ | ���������ɣ���Һ��Ѫ��ɫ | ϡ���ὫFe����ΪFe3�� |
B | ��ͭ�ۼ��뵽1.0 mol��L��1 Fe2(SO4)3��Һ�� | ͭ���ܽ⣬��Һ���� | ��������ͭ���� |
C | ��5 mL 0.005 mol��L��1 FeCl3��Һ��5 mL 0.015 mol��L��1 KSCN��Һ��ϣ��ﵽƽ����ٵμ�4��1 mol��L��1��KCl��Һ | ��Һ��ɫ���� | ����Ӧ��Ũ�ȣ�ƽ�������ƶ� |
D | ��10 mL 0.1 mol��L��1 AgNO3��Һ�еμ�4��0.1 mol��L��1 NaCl��Һ��Ȼ���ٵμ�4��0.1 mol��L��1 Na2S��Һ | ���а�ɫ�������ɣ����к�ɫ�������� | ��ͬ�¶��£�Ag2S���ܶȻ���AgCl��С |
A. A B. B C. C D. D




