��Ŀ����
��һ��ɫ����Һ�����ܺ�Al3����Fe3����Mg2����Na����CO��Cl-��NO�������е������֡���������ʵ�飺
(1)ȡ��������Һ�������������ữ��AgNO 3��Һ���а�ɫ�������ɡ�
(2)��ȡ������Һ�������������ƣ��а�ɫ���������������������Ƶ��������ɰ�ɫ��������������ͼ��ʾ��
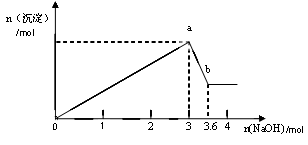
���ƶϣ�
(1)����Һ��һ������__________ ____��һ��������________________��
(2)������Һ��������__________��_________���ѧʽ�������ʻ�϶��ɣ������ʵ�����Ϊ ��
(3) д��ͼ��a b�仯���̵����ӷ���ʽ ��
b�仯���̵����ӷ���ʽ ��
(1)ȡ��������Һ�������������ữ��AgNO 3��Һ���а�ɫ�������ɡ�
(2)��ȡ������Һ�������������ƣ��а�ɫ���������������������Ƶ��������ɰ�ɫ��������������ͼ��ʾ��

���ƶϣ�
(1)����Һ��һ������__________ ____��һ��������________________��
(2)������Һ��������__________��_________���ѧʽ�������ʻ�϶��ɣ������ʵ�����Ϊ ��
(3) д��ͼ��a
 b�仯���̵����ӷ���ʽ ��
b�仯���̵����ӷ���ʽ ����1��Al3����Mg2����Cl- ��Fe3����CO ����д���÷֣�©дһ����1�֣�
��2��AlCl3��MgCl2 �� 1:1 ����д���÷֣�©дһ����1�֣�
��3��Al(OH)3+ OH-="=" AlO2-+ 2H2O
��2��AlCl3��MgCl2 �� 1:1 ����д���÷֣�©дһ����1�֣�
��3��Al(OH)3+ OH-="=" AlO2-+ 2H2O
����ԭ��Һ��ɫ�����ų�Fe3���Ĵ��ڣ�
�ɣ�1����֤������Cl-
�ɣ�2��ͼʾ��֪�����������ܽ⣬һ������Al3����Mg2����ͬʱ�ų�CO32������Al3����Mg2����˫ˮ������ɳ�����
��Mg2����2OH��=Mg(OH)2�� Al3����3OH��=Al(OH)3�� Al(OH)3��OH��=AlO2����2H2O��֪��Mg2����Al3�������ʵ�����Ϊ0.6mol
�ۺ�������Ϣ����Һ�����ٺ���AlCl3��MgCl2 �������ʵ���֮��Ϊ1:1
�ɣ�1����֤������Cl-
�ɣ�2��ͼʾ��֪�����������ܽ⣬һ������Al3����Mg2����ͬʱ�ų�CO32������Al3����Mg2����˫ˮ������ɳ�����
��Mg2����2OH��=Mg(OH)2�� Al3����3OH��=Al(OH)3�� Al(OH)3��OH��=AlO2����2H2O��֪��Mg2����Al3�������ʵ�����Ϊ0.6mol
�ۺ�������Ϣ����Һ�����ٺ���AlCl3��MgCl2 �������ʵ���֮��Ϊ1:1

��ϰ��ϵ�д�
�����Ŀ

 ������������������
������������������