��Ŀ����
ijУ����С���ѧ���������ű���ʳ��ˮ�ķ����ռ���һƽ����ƿ��������ͬʱ�Ʊ���һƽ����ƿ�ı�����ˮ��̽�������������ʵ�顣�밴��Ҫ������������⣺
(1)����ͼ��װ��ʵ��װ�ã�U�ι���ʢ����ɫīˮ��A��B����Һ����ƽ��ƽ����ƿʢ��������ͨ����Һ©����ƽ����ƿ�еμ���������������Һ���۲쵽ʵ�������� �� �������ԭ�� ��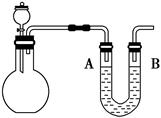
(2)��ͼ��ʾ��ƽ����ƿʢ��������ˮ�����չ����䵽ʢ�б�����ˮ��װ��ʱ���ɹ۲쵽ƽ����ƿ�������ݲ���������һ��ʱ�����Һ��ɫ��dz���������������ԭ���� ������ˮ�в��ٲ�������ʱ��ijѧ��������÷�Ӧ�����������壬��ͬѧ�ɲ�ȡ�ĺ��������� ��
(1)ƽ����ƿ���������ɫ��dz��U�ι�A��ˮλ������B��ˮλ�½���Cl2������������Һ��Ӧ������������ɫ��dz��ͬʱƽ����ƿ������ѹǿ��С��ʹU�ι�A��ˮλ������B��ˮλ�½�
(2)��ˮ�к��д����ᣬ����������ֽ⣬���������������ʹƽ��Cl2��H2O HCl��HClO�����ƶ�����ˮ���������Ӽ��٣�������Һ��ɫ��dz����ƽ����ƿ���ϲ���Ƭ��ȡ���������������ϣ���ȥ����Ƭ���������ǵ�ľ������ƽ����ƿ��ƿ�ڣ�ľ����ȼ
HCl��HClO�����ƶ�����ˮ���������Ӽ��٣�������Һ��ɫ��dz����ƽ����ƿ���ϲ���Ƭ��ȡ���������������ϣ���ȥ����Ƭ���������ǵ�ľ������ƽ����ƿ��ƿ�ڣ�ľ����ȼ
����

 ����������������ϵ�д�
����������������ϵ�д���ʽ̼��ͭ������������;�㷺�Ļ���ԭ�ϡ�
��1����ҵ�Ͽ������Կ�ʴ��Һ����Ҫ�ɷ���Cu2+��Fe2+��Fe3+��H +��Cl?���Ʊ���ʽ̼��ͭ�����Ʊ��������£�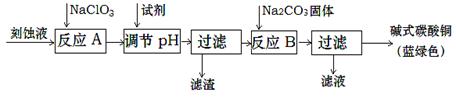
��֪��Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe (OH)2 | Fe (OH)3 |
| ��ʼ����pH | 4.2 | 5.8 | 1.2 |
| ��ȫ����pH | 6.7 | 8.3 | 3.2 |
�������Ƶ������� ��
�ڷ�ӦA�������Һ��pH��ΧӦΪ ��
�۵�һ�ι��˵õ��IJ�Ʒϴ��ʱ������ж��Ѿ�ϴ���� ��
���������ɫ��Ʒ�л���CuO���ʵ�ԭ���� ��
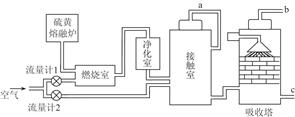
��2��ijѧϰС����ʵ������������ͼ��ʾװ����ȡ������̽�������ʡ�

��ʵ�����ö������̺�Ũ���������ȡ����������������Ҫ��©���� ��
����C��Ʒ����Һ��ɫ���ܷ�֤��������ˮ��Ӧ�IJ�����Ư���ԣ�˵��ԭ�� ����ʱBװ���з�����Ӧ�����ӷ���ʽ��___________ _____��
��д��A��Һ�о���ǿ���������Ļ�ѧʽ ������A��Һ�м���NaHCO3��ĩ����۲쵽�������� ��