��Ŀ����
ijѧϰС�����λͬѧΪ�ⶨ��п��Ƥ�ĶƲ�ĺ�ȣ�����˸��Ե���Ʒ�����������п�Ʋ����������ͬѧ�ķ������������Ὣ��п��Ƥ�����п��Ӧ����ͨ�����������п��������Ȼ������п���ܶ����п�������������������Զ�п��Ƥ���������õ�п��ĺ�ȡ�
��1����ͬѧ�ķ����Ƿ���У�˵�����ɣ� ��
��ͬѧ�ķ�����ͨ���������ϣ�֪��Zn(OH)2�ȿ�������Ҳ����Ӧ��������������·�����

��2������5%������1 L (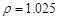 g/cm3
)����ȡ��36.5% (
g/cm3
)����ȡ��36.5% (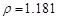 g/cm3
)������
mL
g/cm3
)������
mL
������һλС������
��3����ʹ�õĶ�п��Ƥ������Ϊ28.357g�����Ƶ����պ���������Ϊ40.000g����п��Ƥ�ij�5.00cm����5.00cm��п���ܶ�Ϊ7.14g/cm3����п��ĺ��Ϊ cm����ͬѧ�ķ�����ͨ����ͼ��ʾװ�ã�������п��Ƥ��ϡH2SO4��Ӧ�������������������п��ĺ�ȡ����Ƶö�п��Ƥ����Ϊ18.200g��

��4��ʵ�����ó�������Ϊ ��
��5��������Ũ���ᣬ����п�ĺ�Ȼ� ���ƫ����ƫС��������Ӱ�족����
��6��ʵ�����ͬѧ�ͱ�ͬѧ�Ľ�����бȽϣ��������Ƕ�ͬ�ֶ�п��Ƥ�IJ����������ܴ�����Ϊ˭�ķ������ӿɿ��أ� �����ǣ� ��
��1�������� (1��) FeҲ������ᷴӦ (1��)
��2��118.9 (2��) ��3��0.001 (2��)
��4��������ƽ (2��) ��5��ƫС (2��)
��6���� (1��) ���ķ�������������ˮ����������ɸ��š�(1��)
��������
���������
��2���⣺����36.5% (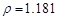 g/cm3
)���������ΪX
g/cm3
)���������ΪX
�������ʲ��䣺1.025*1000*5%=1.181*X*36.5% X=118.9
��3���⣺����������Ϊy
2Fe------- Fe2O3
112 160
y 40.0 y=28
п��ĺ��:(m��-m��)/pп/5*5=(28.357-28)/ 7.14g*5*5= 0.002��cm����Ϊ���������棬���涼��0.001 cm.
��5��ƫС����Ϊ����ӷ�����ʧһ�����ᣬ�������������п��С��
��6�����ķ����Ǹ���������������п������������ˮ��������ɵ����������ܴ�
���㣺�����Ի�ѧʵ��Ϊ����������ʵ����ơ�ʵ������������ͻ�ѧ�����֪ʶ��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�


 �������ۣ�
�������ۣ� ��2013?������һģ��ijѧϰС�����λͬѧΪ�ⶨ��п��Ƥ�ĶƲ�ĺ�ȣ�����˸��Ե���Ʒ�����������п�Ʋ��������
��2013?������һģ��ijѧϰС�����λͬѧΪ�ⶨ��п��Ƥ�ĶƲ�ĺ�ȣ�����˸��Ե���Ʒ�����������п�Ʋ��������