��Ŀ����
ijʵ��С����ȼ�շ������ⶨij�л�����̼�����Ԫ�صĺ���������ֶ������������̽�������ѳ�������Ʒ�����������У�������ͭ���������ڸ�����������Ʒȫ��������Ϊˮ�Ͷ�����̼��Ȼ��ֱ�ⶨ���ɵ�ˮ�Ͷ�����̼��ʵ������õ���װ����ͼ��ʾ������Aװ�ÿ����ظ�ʹ�ã�
��ش��������⣺
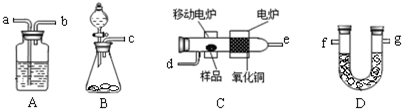
��1���밴������������ʵ��װ��______��______��______��d��______��______��______��g���������ӿڱ����д����
��2��Bװ������O2ʱ���õ�ҩƷ��______��ʵ���У���ʼ��Cװ�ü���֮ǰ��Ҫͨһ��ʱ���������Ŀ����______��ֹͣ���Ⱥ�ҲҪ��ͨһ��ʱ���������Ŀ����______��
��3����֪ȡ2.3g����ƷX��������ʵ�飬���ⶨAװ������2.7g��Dװ������4.4g���������X���ʵ�ʵ��ʽ______��
��4����С��ͬѧ��һ��ʵ���ã�2.3g��X����������Ʒ�Ӧ�ɷų�560mL H2���ѻ���ɱ�״���£�������֪X����ֻ��һ�������ţ��������Ϻ�ѧ�����ֽ���������̽��ʵ�飺
ʵ��һ��X��һ�������¿ɴ��������������л���Y��
ʵ�����X��Y��Ũ������������������л���Z��
���д��ʵ����з�Ӧ�Ļ�ѧ����ʽ______��
�ڳ�ȥZ�л��е�Y������Լ�����Ҫ������______��______��
��5������֪������2.3gҺ̬X����������ȫȼ�����ɶ�����̼�����Һ̬ˮʱ�ɷų�68.35kJ��������д��X��������ȼ�յ��Ȼ�ѧ����ʽ______��

��ش��������⣺
��1���밴������������ʵ��װ��______��______��______��d��______��______��______��g���������ӿڱ����д����
��2��Bװ������O2ʱ���õ�ҩƷ��______��ʵ���У���ʼ��Cװ�ü���֮ǰ��Ҫͨһ��ʱ���������Ŀ����______��ֹͣ���Ⱥ�ҲҪ��ͨһ��ʱ���������Ŀ����______��
��3����֪ȡ2.3g����ƷX��������ʵ�飬���ⶨAװ������2.7g��Dװ������4.4g���������X���ʵ�ʵ��ʽ______��
��4����С��ͬѧ��һ��ʵ���ã�2.3g��X����������Ʒ�Ӧ�ɷų�560mL H2���ѻ���ɱ�״���£�������֪X����ֻ��һ�������ţ��������Ϻ�ѧ�����ֽ���������̽��ʵ�飺
ʵ��һ��X��һ�������¿ɴ��������������л���Y��
ʵ�����X��Y��Ũ������������������л���Z��
���д��ʵ����з�Ӧ�Ļ�ѧ����ʽ______��
�ڳ�ȥZ�л��е�Y������Լ�����Ҫ������______��______��
��5������֪������2.3gҺ̬X����������ȫȼ�����ɶ�����̼�����Һ̬ˮʱ�ɷų�68.35kJ��������д��X��������ȼ�յ��Ȼ�ѧ����ʽ______��
��1��ʵ���ԭ�����Ȳ����������������ͨ�뷢��װ�ã�Ȼ��ͨ��Aװ�òⶨˮ��Dװ�òⶨ������̼������������������ʵ��װ��˳��Ϊ��c��a��b��d��e��a��b��g��
�ʴ�Ϊ��c��a��b��e��a��b��
��2��Bװ����ȡO2�ǹ�̬ҩƷ��Һ�巴Ӧ�����õ�ҩƷ��H2O2��MnO2��Na2O2��H2O��ʵ��ǰ�����ų�װ���еĶ�����̼��ˮ�������Է����������Կ�ʼ��Cװ�ü���֮ǰ��Ҫͨһ��ʱ����������ų�װ���еĶ�����̼��ˮ�����ȣ�ֹͣ���Ⱥ�װ���л����һЩȼ�����ɵĶ�����̼��ˮ������Ϊȷ��������̼��ˮ���������ų���Ӧ��ͨһ��ʱ���������
�ʴ�Ϊ��H2O2��MnO2��Na2O2��H2O���ų�װ���еĶ�����̼��ˮ�����ȣ���ȼ�����ɵĶ�����̼��ˮ���������ų�������ȫ���գ�
��3��Ũ��������2.7g��ȼ�����ɵ�ˮΪ2.7g����ˮ�����ʵ���Ϊ0.15mol�����к���Ԫ�ص�����Ϊ0.3g����ʯ������4.4g����ȼ�����ɵ�CO2ˮ4.4g����CO2�����ʵ���Ϊ0.1mol�����к���̼Ԫ�ص�����Ϊ1.2g����������̼Ԫ�غ���Ԫ�ص�����֮��Ϊ1.2g+0.3g=1.5g��С���л��������2.3g��˵���л�����һ��������Ԫ�أ�����������Ϊ2.3g-1.5g=0.8g����Ԫ�ص����ʵ���Ϊ0.05mol�����л�����C��H��O����Ԫ�ص����ʵ���֮��Ϊ2��6��1������X���ʵ�ʵ��ʽΪC2H6O��
�ʴ�Ϊ��C2H6O��
��4��X��ʵ��ʽC2H6O��̼ԭ���Ѿ����ͣ����Է���ʽΪC2H6O���Լ�2.3g��0��1mol��X����������Ʒ�Ӧ�ɷų�560mL��0.25molH2������֪X����ֻ��һ�������ţ�˵��X���Ҵ����Ҵ������õ����ᣬ
�����������Ҵ�����������Ӧ�õ�����������ˮ������ʽΪ��CH3COOH+C2H5OH CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+C2H5OH CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
����������̼������Һ��Ӧ����������������ˮ����̼������Һ����Ӧ�����ӡ����룬���Գ�ȥ���������л��е�����������Լ��DZ���Na2CO3��Һ���÷�Һ�ķ������룬���������Ƿ�Һ©����
�ʴ�Ϊ������Na2CO3��Һ����Һ©����
��5��2.3g�Ҵ������ʵ���Ϊ
=0.05mol������������ȫȼ�����ɶ�����̼�����Һ̬ˮʱ�ɷų�68.35kJ��������1mol�Ҵ���ȫ��Ӧ����68.35kJ��
=1367kJ���Ȼ�ѧ����ʽΪ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l����H=-1367kJ/mol��
�ʴ�Ϊ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l����H=-1367kJ/mol��
�ʴ�Ϊ��c��a��b��e��a��b��
��2��Bװ����ȡO2�ǹ�̬ҩƷ��Һ�巴Ӧ�����õ�ҩƷ��H2O2��MnO2��Na2O2��H2O��ʵ��ǰ�����ų�װ���еĶ�����̼��ˮ�������Է����������Կ�ʼ��Cװ�ü���֮ǰ��Ҫͨһ��ʱ����������ų�װ���еĶ�����̼��ˮ�����ȣ�ֹͣ���Ⱥ�װ���л����һЩȼ�����ɵĶ�����̼��ˮ������Ϊȷ��������̼��ˮ���������ų���Ӧ��ͨһ��ʱ���������
�ʴ�Ϊ��H2O2��MnO2��Na2O2��H2O���ų�װ���еĶ�����̼��ˮ�����ȣ���ȼ�����ɵĶ�����̼��ˮ���������ų�������ȫ���գ�
��3��Ũ��������2.7g��ȼ�����ɵ�ˮΪ2.7g����ˮ�����ʵ���Ϊ0.15mol�����к���Ԫ�ص�����Ϊ0.3g����ʯ������4.4g����ȼ�����ɵ�CO2ˮ4.4g����CO2�����ʵ���Ϊ0.1mol�����к���̼Ԫ�ص�����Ϊ1.2g����������̼Ԫ�غ���Ԫ�ص�����֮��Ϊ1.2g+0.3g=1.5g��С���л��������2.3g��˵���л�����һ��������Ԫ�أ�����������Ϊ2.3g-1.5g=0.8g����Ԫ�ص����ʵ���Ϊ0.05mol�����л�����C��H��O����Ԫ�ص����ʵ���֮��Ϊ2��6��1������X���ʵ�ʵ��ʽΪC2H6O��
�ʴ�Ϊ��C2H6O��
��4��X��ʵ��ʽC2H6O��̼ԭ���Ѿ����ͣ����Է���ʽΪC2H6O���Լ�2.3g��0��1mol��X����������Ʒ�Ӧ�ɷų�560mL��0.25molH2������֪X����ֻ��һ�������ţ�˵��X���Ҵ����Ҵ������õ����ᣬ
�����������Ҵ�����������Ӧ�õ�����������ˮ������ʽΪ��CH3COOH+C2H5OH
 CH3COOC2H5+H2O��
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+C2H5OH
 CH3COOC2H5+H2O��
CH3COOC2H5+H2O������������̼������Һ��Ӧ����������������ˮ����̼������Һ����Ӧ�����ӡ����룬���Գ�ȥ���������л��е�����������Լ��DZ���Na2CO3��Һ���÷�Һ�ķ������룬���������Ƿ�Һ©����
�ʴ�Ϊ������Na2CO3��Һ����Һ©����
��5��2.3g�Ҵ������ʵ���Ϊ
| 2.3g |
| 46g/mol |
| 1mol |
| 0.05mol |
�ʴ�Ϊ��C2H5OH��l��+3O2��g��=2CO2��g��+3H2O��l����H=-1367kJ/mol��

��ϰ��ϵ�д�
�����Ŀ
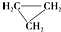 ��CH3-CH�TCH2��Ϊͬ���칹��
��CH3-CH�TCH2��Ϊͬ���칹��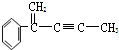 �����жԸ��л���ṹ����������ȷ���ǣ�������
�����жԸ��л���ṹ����������ȷ���ǣ�������