��Ŀ����
CO��CO2�����Ժϳɼ״���CO��2H2��CH3OH����CO2��3H2��CH3OH��H2O
CO��CO2��H2��ͨ�����з�Ӧ�Ʊ���
A��CH4��H2O(g)��CO��3H2����B��CO��H2O(g)��CO2��H2
��ӦA�IJ���ϳɼ״�ʱH2��������ӦB�IJ���ϳɼ״�ʱH2���㣮Ϊ�˳������ԭ�Ͽɽ�������Ӧ�IJ�����ʹ�ã�
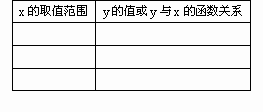
(1)��ӦA�ͷ�ӦB�IJ��������ϵ���ѱ���(��ͬ��ͬѹ����������ȱ�ʾ)
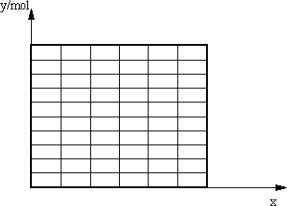
(2)��ȡA�IJ�����B�IJ���������67.2 L(��״��)������CO���������Ϊx���õ��״������ʵ���y(������)��CO��������仯�ĺ�����ϵʽ������ͼ��(�ٶ�CO����H2��Ӧ��CO��ȫ��Ӧ��CO2����H2��Ӧ)��
�𰸣�
������

������
����(1)4��1(2��);
����(2)(2)x��0.2��y��0.9 mol��(2��)
����0��x��0.2��y��2x��0.5(3��)0.2��x��0.25��y��1.5��3x(3��)

������ͼ(2��)

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�
�����Ŀ