��Ŀ����
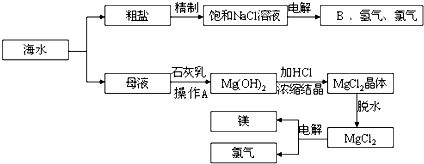
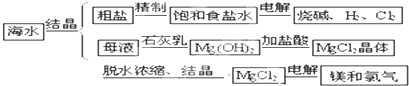
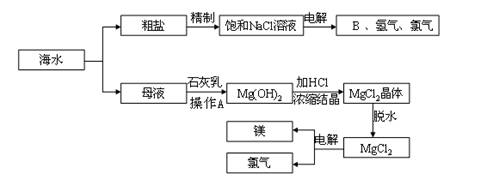
��ˮ����ȡ֮�����Ļ�ѧ��Դ���Ӻ�ˮ�п���ȡ���ֻ���ԭ�ϣ�ͼ��ij�����Ժ�ˮ��Դ���ۺ����õ�ʾ��ͼ��
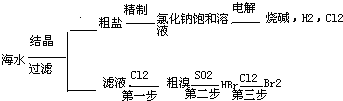
��1�������к�Ca2+��Mg2+��SO
�����ʣ�����ʱ�����Լ�Ϊ�������BaCl2��Һ����NaOH��Һ����Na2CO3��Һ�������Լ�˳����
A���ڢۢ٢�B���ڢۢܢ�C���ۢڢ٢�D���٢ܢڢ�
��2����ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫�Ϸ�����Ӧ����
��3����ˮ��ȡʳ�κ��ĸҺ����K+��Na+��Mg2+�������ӣ���ĸҺ������ʯ������ʲô���ã�
��4��д���Ӻ�ˮ����ȡBr2�ĵ�һ����Ӧ�����ӷ���ʽ
��5��Ҫʹ�������ɵ�Br2��ˮ��Һ�з��룬���ȼ���
A��ˮ B�����Ȼ�̼ C���ƾ� D������ E����Һ��

��1�������к�Ca2+��Mg2+��SO
2- 4 |
B
B
A���ڢۢ٢�B���ڢۢܢ�C���ۢڢ٢�D���٢ܢڢ�
��2����ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫�Ϸ�����Ӧ����
����
����
���壬���Դ���������ĵ缫������ҺpH���
���
�����������䡱��С��������3����ˮ��ȡʳ�κ��ĸҺ����K+��Na+��Mg2+�������ӣ���ĸҺ������ʯ������ʲô���ã�
ʹþ��������������þ����
ʹþ��������������þ����
��4��д���Ӻ�ˮ����ȡBr2�ĵ�һ����Ӧ�����ӷ���ʽ
Cl2+2Br-=Br2+2Cl-
Cl2+2Br-=Br2+2Cl-
���ڶ�����Ӧ�Ļ�ѧ����ʽBr2 +SO2 +2H2O=H2SO4 +2HBr
Br2 +SO2 +2H2O=H2SO4 +2HBr
����5��Ҫʹ�������ɵ�Br2��ˮ��Һ�з��룬���ȼ���
B
B
����E
E
����ѡ������ѡ����գ�A��ˮ B�����Ȼ�̼ C���ƾ� D������ E����Һ��
��������1���ȼ�������Ȼ�����ȥ��������ӣ�Ȼ�����NaOH��Һ��ʹþ����ת��ΪMg��OH��2������Na2CO3��Һ��ʹ��Һ�б����ӡ�������ת��ΪCaCO3��Ϊ�˳������ʣ������������ȥ������̼���ơ��������ƣ�
��2����ⱥ��ʳ��ˮ��Һʱ�������������ӷŵ����������������������ӷŵ�����������ͬʱ���������������������ɣ���������������Ũ�ȵı仯�ж���ҺPH�ı仯��
��3������ʯ�����ʹþ��������Mg��OH��2������
��4���������������ԣ����������������ɵ����壻����������л�ԭ�ԣ��ܹ����嵥�����������
��5��ͨ����ȡ�����������Һ�з��룮
��2����ⱥ��ʳ��ˮ��Һʱ�������������ӷŵ����������������������ӷŵ�����������ͬʱ���������������������ɣ���������������Ũ�ȵı仯�ж���ҺPH�ı仯��
��3������ʯ�����ʹþ��������Mg��OH��2������
��4���������������ԣ����������������ɵ����壻����������л�ԭ�ԣ��ܹ����嵥�����������
��5��ͨ����ȡ�����������Һ�з��룮
����⣺��1������������Ȼ�����ȥ��������ӣ�Ȼ������NaOH��Һ��ʹþ����ת��ΪMg��OH��2�������Na2CO3��Һ��ʹ��Һ�б����ӡ�������ת��ΪBaCO3��CaCO3�����˺�������������ȥ������̼���ơ��������ƣ�������ȷ˳��Ϊ���ڢۢܢ٣�
��ѡB��
��2����ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫��������ʧ���ӷ���������Ӧ���缫��ӦʽΪ�������ķ�ӦΪ2Cl--2e��Cl2�������������ӵõ�������������ͬʱ���������������������ӣ���Һ�ʼ��ԣ�pH���
�ʴ�Ϊ�����������
��3����ĸҺ������ʯ���飬�ܹ�ʹþ��������������þ������
�ʴ�Ϊ��ʹþ��������������þ������
��4�������������������ԣ����������������ɵ����壺Cl2+2Br-=Br2+2Cl-���嵥���ܹ��������������������ᣬ
�ʴ�Ϊ��Cl2+2Br-=Br2+2Cl-��Br2 +SO2 +2H2O=H2SO4 +2HBr��
��5��Ҫʹ�������ɵ�Br2��ˮ��Һ�з��룬���ȼ����л��ܼ����Ȼ�̼��ʹ��Һ�ֲ㣬Ȼ��ͨ����Һ���������������Ȼ�̼��Һ�����������嵥�ʣ�
�ʴ�Ϊ��B��E��
��ѡB��
��2����ⱥ��ʳ��ˮʱ�����Դ���������ĵ缫��������ʧ���ӷ���������Ӧ���缫��ӦʽΪ�������ķ�ӦΪ2Cl--2e��Cl2�������������ӵõ�������������ͬʱ���������������������ӣ���Һ�ʼ��ԣ�pH���
�ʴ�Ϊ�����������
��3����ĸҺ������ʯ���飬�ܹ�ʹþ��������������þ������
�ʴ�Ϊ��ʹþ��������������þ������
��4�������������������ԣ����������������ɵ����壺Cl2+2Br-=Br2+2Cl-���嵥���ܹ��������������������ᣬ
�ʴ�Ϊ��Cl2+2Br-=Br2+2Cl-��Br2 +SO2 +2H2O=H2SO4 +2HBr��
��5��Ҫʹ�������ɵ�Br2��ˮ��Һ�з��룬���ȼ����л��ܼ����Ȼ�̼��ʹ��Һ�ֲ㣬Ȼ��ͨ����Һ���������������Ȼ�̼��Һ�����������嵥�ʣ�
�ʴ�Ϊ��B��E��
���������⿼����ԭ���ԭ���͵���ԭ�������ӵ�֪ʶ�㣬�ѶȽϴ�ע��ȼ�ϵ���е缫��Ӧʽ����дҪ��ϵ������Һ������ԣ���ʹȼ�Ϻ���������ͬ���������Һ��ͬ��缫��ӦʽҲ��ͬ��Ϊ�״���

��ϰ��ϵ�д�
�����Ŀ