��Ŀ����
��10�֣�A��B��C��D��E��F�����ֶ���������Ԫ�أ����ǵ�ԭ������������������A��D��C��F�ֱ���ͬһ����Ԫ�ء�A��C��Ԫ�ؿ��γ�ԭ�Ӹ���֮��Ϊ2�U:1��1�U1�ͻ����BԪ�ص��������������ڲ��������2����EԪ�ص�������������������Ӳ�����FԪ�ص������������Ǵ�����������0.75������ش�
��1��D��F�γ�D2F�ĵ���ʽΪ______________��A��C��D����Ԫ����ɵĻ����ﺬ�л�ѧ����������________________��
��2����E��F����Ԫ����ɵĻ�����1 mol����A��C��D����Ԫ����ɵĻ��������Һ������Ӧ�����ĺ������ʵ������ֵΪ___________ mol��
��3��A��C��F���γɵļס���������������18�����ӣ�����10�����ӣ����Ǿ�Ϊ��һ��˫ԭ�������ӣ�������ҷ�Ӧ�����ӷ���ʽΪ_________________________________ ��
��4����ҵ���ڸ��µ������£��� ����A2C��BC��Ӧ��ȡ����A2���ڵ�����Ģ������ܱ������зֱ����1 mol A2C��1 mol BC��2 mol A2C��2 mol BC��һ�������£���ַ�Ӧ��ֱ�ﵽƽ�⣨�������¶���ͬ��������˵����ȷ����________________ ��
����A2C��BC��Ӧ��ȡ����A2���ڵ�����Ģ������ܱ������зֱ����1 mol A2C��1 mol BC��2 mol A2C��2 mol BC��һ�������£���ַ�Ӧ��ֱ�ﵽƽ�⣨�������¶���ͬ��������˵����ȷ����________________ ��
A���ﵽƽ������Ҫ��ʱ�䣺�� B���ﵽƽ���A2C��ת���ʣ���
C���ﵽƽ���BC�����ʵ������� D���ﵽƽ���A2�������������
E���ﵽƽ������ջ�ų����������� F���ﵽƽ�����ϵ��ƽ����Է�����������
��1��D��F�γ�D2F�ĵ���ʽΪ______________��A��C��D����Ԫ����ɵĻ����ﺬ�л�ѧ����������________________��
��2����E��F����Ԫ����ɵĻ�����1 mol����A��C��D����Ԫ����ɵĻ��������Һ������Ӧ�����ĺ������ʵ������ֵΪ___________ mol��
��3��A��C��F���γɵļס���������������18�����ӣ�����10�����ӣ����Ǿ�Ϊ��һ��˫ԭ�������ӣ�������ҷ�Ӧ�����ӷ���ʽΪ_________________________________ ��
��4����ҵ���ڸ��µ������£���
 ����A2C��BC��Ӧ��ȡ����A2���ڵ�����Ģ������ܱ������зֱ����1 mol A2C��1 mol BC��2 mol A2C��2 mol BC��һ�������£���ַ�Ӧ��ֱ�ﵽƽ�⣨�������¶���ͬ��������˵����ȷ����________________ ��
����A2C��BC��Ӧ��ȡ����A2���ڵ�����Ģ������ܱ������зֱ����1 mol A2C��1 mol BC��2 mol A2C��2 mol BC��һ�������£���ַ�Ӧ��ֱ�ﵽƽ�⣨�������¶���ͬ��������˵����ȷ����________________ ��A���ﵽƽ������Ҫ��ʱ�䣺�� B���ﵽƽ���A2C��ת���ʣ���
C���ﵽƽ���BC�����ʵ������� D���ﵽƽ���A2�������������
E���ﵽƽ������ջ�ų����������� F���ﵽƽ�����ϵ��ƽ����Է�����������
��1��Na2S ����ʽ���ԣ������Ӽ������ۼ���(��2��)
��2��8 mol ��2�֣�
��3��HS�� + OH�� �� S2�� + H2O��2�֣�
��4��A ��B��©ѡ��1�֣���ѡ��ѡ���÷֣�2�֣�
��2��8 mol ��2�֣�
��3��HS�� + OH�� �� S2�� + H2O��2�֣�
��4��A ��B��©ѡ��1�֣���ѡ��ѡ���÷֣�2�֣�
��

��ϰ��ϵ�д�
�����Ŀ

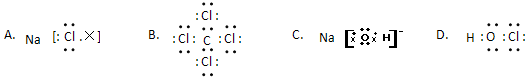
 Z+W
Z+W
 �������ͨ��������ˮ�У���ʹ���屻��ȫ����������Ӧͬʱͨ���״���µĿ��� L������������Ϊ��N2��O2�������4��1��
�������ͨ��������ˮ�У���ʹ���屻��ȫ����������Ӧͬʱͨ���״���µĿ��� L������������Ϊ��N2��O2�������4��1��