��Ŀ����
ij�л���X��C12H13O6Br�������к��ж��ֹ����ţ���ṹ��ʽΪ ������I����Ϊδ֪���ֵĽṹ��
������I����Ϊδ֪���ֵĽṹ��
Ϊ�Ʋ�X�ķ��ӽṹ����������ͼ��ת����

��֪��E��ˮ��Һ�е���FeCl3����Һ������ɫ��Ӧ��G��M������NaHCO3��Һ��Ӧ
��1��M�Ľṹ��ʽΪ
��2��E���ܷ����ķ�Ӧ�У�ѡ����ţ�
A���ӳɷ�Ӧ B����ȥ��Ӧ C��������Ӧ D��ȡ����Ӧ
��3����Bת����D�Ļ�ѧ����ʽ��
 ��
��
��4��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ������д��G����������Ӧ�Ļ�ѧ����ʽ
 ��
��
��5����֪��X���ӽṹ�У�I�ﺬ������FeCl3��Һ������ɫ��Ӧ�Ĺ����ţ���E�����б����ϵ�һ�ȴ���ֻ��һ�֣���X�Ľṹ��ʽ��
 ��
��
��6��д������һ�ַ�������������G��ͬ���칹�壺
a���ܷ���������Ӧ b������NaHCO3��Ӧ c��1mol��������������Na��Ӧ�ų�1.5molH2
 ��
��
 ������I����Ϊδ֪���ֵĽṹ��
������I����Ϊδ֪���ֵĽṹ��Ϊ�Ʋ�X�ķ��ӽṹ����������ͼ��ת����

��֪��E��ˮ��Һ�е���FeCl3����Һ������ɫ��Ӧ��G��M������NaHCO3��Һ��Ӧ
��1��M�Ľṹ��ʽΪ
HOOC-COOH
HOOC-COOH
��G�������������ŵ��������Ȼ��ʹ��ǻ�
�Ȼ��ʹ��ǻ�
����2��E���ܷ����ķ�Ӧ�У�ѡ����ţ�
B
B
��A���ӳɷ�Ӧ B����ȥ��Ӧ C��������Ӧ D��ȡ����Ӧ
��3����Bת����D�Ļ�ѧ����ʽ��


��4��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ������д��G����������Ӧ�Ļ�ѧ����ʽ


��5����֪��X���ӽṹ�У�I�ﺬ������FeCl3��Һ������ɫ��Ӧ�Ĺ����ţ���E�����б����ϵ�һ�ȴ���ֻ��һ�֣���X�Ľṹ��ʽ��


��6��д������һ�ַ�������������G��ͬ���칹�壺
a���ܷ���������Ӧ b������NaHCO3��Ӧ c��1mol��������������Na��Ӧ�ų�1.5molH2


��������E��ˮ��Һ�е���FeCl3��Һ����ɫ��Ӧ��˵��X�к��б����������л���X�ķ���ʽC12H13O6Br�������Ͷ�Ϊ
=6�������֪���ֵĽṹ�����ж�X�����г��˱���������̼��˫���⣬û�����������ͼ���
��ת����ϵ��B������������M��M�ķ���ʽΪC2H2O4����֪BΪ�Ҷ�����DΪ�Ҷ�ȩ��MΪ�Ҷ��ᣬ
���ڷ���±������ˮ�⼫�����ѣ�II�к���Brԭ�ӣ����II��Ӧ����2��̼ԭ�Ӻ�һ��-Br����-Brλ�����ˣ�����ò����Ҷ��������ɣ�5���п�֪��I�ﺬ������FeCl3��Һ������ɫ��Ӧ�Ĺ����ţ�E�ı����ϵ�һ�ȴ���ֻ��һ�֣�����л���X�ķ���ʽ����֪���ֽṹ��֪��E�Ľṹ��ʽΪ�� �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ�� ����G�Ľṹ��ʽΪ��
����G�Ľṹ��ʽΪ�� ���ݴ˽��
���ݴ˽��
| 2��12+2-13-1 |
| 2 |
��ת����ϵ��B������������M��M�ķ���ʽΪC2H2O4����֪BΪ�Ҷ�����DΪ�Ҷ�ȩ��MΪ�Ҷ��ᣬ
���ڷ���±������ˮ�⼫�����ѣ�II�к���Brԭ�ӣ����II��Ӧ����2��̼ԭ�Ӻ�һ��-Br����-Brλ�����ˣ�����ò����Ҷ��������ɣ�5���п�֪��I�ﺬ������FeCl3��Һ������ɫ��Ӧ�Ĺ����ţ�E�ı����ϵ�һ�ȴ���ֻ��һ�֣�����л���X�ķ���ʽ����֪���ֽṹ��֪��E�Ľṹ��ʽΪ��
 �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ�� ����G�Ľṹ��ʽΪ��
����G�Ľṹ��ʽΪ�� ���ݴ˽��
���ݴ˽������⣺��E��ˮ��Һ�е���FeCl3��Һ����ɫ��Ӧ��˵��X�к��б����������л���X�ķ���ʽC12H13O6Br�������Ͷ�Ϊ
=6�������֪���ֵĽṹ�����ж�X�����г��˱���������̼��˫���⣬û�����������ͼ���
��ת����ϵ��B������������M��M�ķ���ʽΪC2H2O4����֪BΪ�Ҷ�����DΪ�Ҷ�ȩ��MΪ�Ҷ��ᣬ
���ڷ���±������ˮ�⼫�����ѣ�II�к���Brԭ�ӣ����II��Ӧ����2��̼ԭ�Ӻ�һ��-Br����-Brλ�����ˣ�����ò����Ҷ��������ɣ�5���п�֪��I�ﺬ������FeCl3��Һ������ɫ��Ӧ�Ĺ����ţ�E�ı����ϵ�һ�ȴ���ֻ��һ�֣�����л���X�ķ���ʽ����֪���ֽṹ��֪��E�Ľṹ��ʽΪ�� �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ�� ����G�Ľṹ��ʽΪ��
����G�Ľṹ��ʽΪ�� ��
��
��1��MΪ�Ҷ��ᣬ�ṹ��ʽΪHOOC-COOH��G�Ľṹ��ʽΪ ������G�к����Ȼ��ʹ��ǻ����ʴ�Ϊ��HOOC-COOH���Ȼ������ǻ���
������G�к����Ȼ��ʹ��ǻ����ʴ�Ϊ��HOOC-COOH���Ȼ������ǻ���
��2��E�Ľṹ��ʽΪ ��E�ܷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ�����ܷ�����ȥ��Ӧ����ѡB��
��E�ܷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ�����ܷ�����ȥ��Ӧ����ѡB��
��3���Ҷ������������������Ҷ�ȩ��ˮ����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4��G�Ľṹ��ʽΪ�� ��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����˵���������к���̼̼˫�������ݷ���ʽ֪��G������ȥ��Ӧ����Ӧ����ʽΪ��
��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����˵���������к���̼̼˫�������ݷ���ʽ֪��G������ȥ��Ӧ����Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��5��������������֪��X�Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6��G�Ľṹ��ʽΪ�� ��G��ͬ���칹�壬a���ܷ���������Ӧ��˵������ȩ����b������NaHCO3��Ӧ˵�������Ȼ���c��1mol��������������Na��Ӧ�ų�1.5molH2��˵��������һ�����ǻ���������ṹ��ʽΪ
��G��ͬ���칹�壬a���ܷ���������Ӧ��˵������ȩ����b������NaHCO3��Ӧ˵�������Ȼ���c��1mol��������������Na��Ӧ�ų�1.5molH2��˵��������һ�����ǻ���������ṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
| 2��12+2-13-1 |
| 2 |
��ת����ϵ��B������������M��M�ķ���ʽΪC2H2O4����֪BΪ�Ҷ�����DΪ�Ҷ�ȩ��MΪ�Ҷ��ᣬ
���ڷ���±������ˮ�⼫�����ѣ�II�к���Brԭ�ӣ����II��Ӧ����2��̼ԭ�Ӻ�һ��-Br����-Brλ�����ˣ�����ò����Ҷ��������ɣ�5���п�֪��I�ﺬ������FeCl3��Һ������ɫ��Ӧ�Ĺ����ţ�E�ı����ϵ�һ�ȴ���ֻ��һ�֣�����л���X�ķ���ʽ����֪���ֽṹ��֪��E�Ľṹ��ʽΪ��
 �����Ͽ�֪X�Ľṹ��ʽΪ��
�����Ͽ�֪X�Ľṹ��ʽΪ�� ����G�Ľṹ��ʽΪ��
����G�Ľṹ��ʽΪ�� ��
����1��MΪ�Ҷ��ᣬ�ṹ��ʽΪHOOC-COOH��G�Ľṹ��ʽΪ
 ������G�к����Ȼ��ʹ��ǻ����ʴ�Ϊ��HOOC-COOH���Ȼ������ǻ���
������G�к����Ȼ��ʹ��ǻ����ʴ�Ϊ��HOOC-COOH���Ȼ������ǻ��� ��2��E�Ľṹ��ʽΪ
 ��E�ܷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ�����ܷ�����ȥ��Ӧ����ѡB��
��E�ܷ����ӳɷ�Ӧ��ȡ����Ӧ��������Ӧ�����ܷ�����ȥ��Ӧ����ѡB����3���Ҷ������������������Ҷ�ȩ��ˮ����Ӧ����ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����4��G�Ľṹ��ʽΪ��
 ��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����˵���������к���̼̼˫�������ݷ���ʽ֪��G������ȥ��Ӧ����Ӧ����ʽΪ��
��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����˵���������к���̼̼˫�������ݷ���ʽ֪��G������ȥ��Ӧ����Ӧ����ʽΪ�� ��
���ʴ�Ϊ��
 ��
����5��������������֪��X�Ľṹ��ʽΪ��
 ��
���ʴ�Ϊ��
 ��
����6��G�Ľṹ��ʽΪ��
 ��G��ͬ���칹�壬a���ܷ���������Ӧ��˵������ȩ����b������NaHCO3��Ӧ˵�������Ȼ���c��1mol��������������Na��Ӧ�ų�1.5molH2��˵��������һ�����ǻ���������ṹ��ʽΪ
��G��ͬ���칹�壬a���ܷ���������Ӧ��˵������ȩ����b������NaHCO3��Ӧ˵�������Ȼ���c��1mol��������������Na��Ӧ�ų�1.5molH2��˵��������һ�����ǻ���������ṹ��ʽΪ ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϺͺϳɣ���Ŀ��Ϊ�ۺϣ���һ�����Ѷȣ�����ʱע��������йؼ���Ϣ���������������ϵķ����ƶϣ�ͬʱ����ѧ����������ͽ�������������

��ϰ��ϵ�д�
 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�
�����Ŀ

 Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ
Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ

 �������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ
�������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ ij�л���X�ļ���ʽΪ��
ij�л���X�ļ���ʽΪ��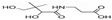 �������л���X��˵����ȷ���ǣ�������
�������л���X��˵����ȷ���ǣ�������