��Ŀ����
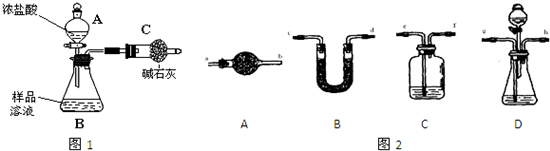
��12�֣���֪ij���Na2CO3�������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣
��Ҫʵ�鲽�����£�
�ٰ�ͼ��װ�����������װ�õ������ԣ�
�ڽ�a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ��
�۳���ʢ�м�ʯ�ҵ�U�ܵ��������õ�b g��
�ܴӷ�Һ©������6 mol��L-1�����ᣬֱ�����ٲ�������ʱΪֹ��
�ݴӵ���A����������һ�����Ŀ�����
���ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�c g��
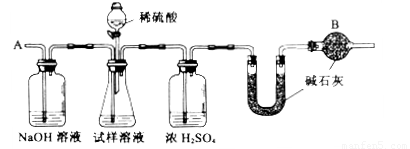
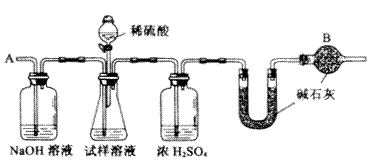
���ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊd g��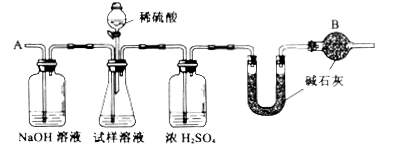
����պͻش����⣺
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��
��2��װ���и����B��������
��3���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ���� ����ƫ�ߡ�ƫ�ͻ䣩
��4������ݵ�Ŀ����
��5������ߵ�Ŀ����
��6���������д�������������ļ���ʽΪ
��12�֣�
����Ʒ�أ������ᣨ2�֣�
�Ʒ�ֹ�����е�CO2��ˮ��������U���У�2�֣�
��ƫ�ߣ�2�֣�
�Ȱѷ�Ӧ������CO2ȫ������U���У�2�֣�
���жϷ�Ӧ������CO2�Ƿ�ȫ���ų�������U���еļ�ʯ�����գ�2�֣�
��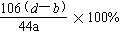 ��2�֣�
��2�֣�
����

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�