��Ŀ����
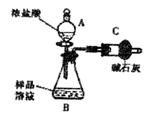
��֪ij������Ʒ�к���NaCl���ʣ�Ϊ�ⶨ��Ʒ�д����������������ͬѧ����ͼ1��ʾװ�ü��Լ�����ʵ��
���г������ԣ���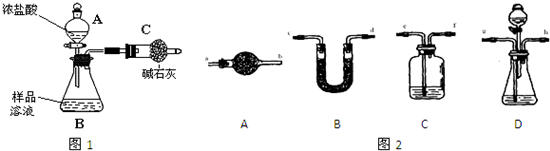
��1������A��B�����Ʒֱ���
��2����ͬѧ��ʵ��������������Ҫ�������£�
��
�ڽ�a g������������B�У�����������ˮ�ܽ⣬�õ���Ʒ��Һ��
�۳���ʢ�м�ʯ�ҵĸ���ܵ�����Ϊb g��
�ܴ�����A�е���Ũ���ᣬֱ�����ٲ�������ʱΪֹ��
���ٴγ���ʢ�м�ʯ�ҵĸ���ܵ�����Ϊc g��
��3����ͬѧ��������ʵ����õ���Ʒ��Na2CO3������������
���ú�a��b��c��ʽ�ӱ�ʾ����
��4����ͬѧ��Ϊ��ͬѧ��ʵ��װ����ƺ�ʹ��ҩƷ�϶���ȱ�ݣ��ᵼ�²�õ�Na2CO3����������ƫ�ߣ�����ͬѧ����Ϊ��ͬѧ��ʵ��װ�û�ʹ��õĽ��ƫ�ͣ���ͬѧ��Ϊ���ƫ�͵�ԭ���ǣ�
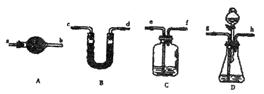
��5����Ҫ����ҡ���ͬѧָ���ļ�ͬѧʵ���е����⣬�ɶԼ�ͬѧ��ʵ��װ�ú�ʵ��ҩƷ�����ʵ��ĸĶ����밴����˳��ѡ����ͼ2��ʾ����������±������ô�д��ĸ�����������������ظ�ʹ�ã��г������ԣ�
���г������ԣ���

��1������A��B�����Ʒֱ���
��Һ©��
��Һ©��
����ƿ
��ƿ
����2����ͬѧ��ʵ��������������Ҫ�������£�
��
���װ�������ԣ����Ӻ�װ��
���װ�������ԣ����Ӻ�װ��
���ڽ�a g������������B�У�����������ˮ�ܽ⣬�õ���Ʒ��Һ��
�۳���ʢ�м�ʯ�ҵĸ���ܵ�����Ϊb g��
�ܴ�����A�е���Ũ���ᣬֱ�����ٲ�������ʱΪֹ��
���ٴγ���ʢ�м�ʯ�ҵĸ���ܵ�����Ϊc g��
��3����ͬѧ��������ʵ����õ���Ʒ��Na2CO3������������
| 53(b-a) |
| 22a |
| 53(b-a) |
| 22a |
��4����ͬѧ��Ϊ��ͬѧ��ʵ��װ����ƺ�ʹ��ҩƷ�϶���ȱ�ݣ��ᵼ�²�õ�Na2CO3����������ƫ�ߣ�����ͬѧ����Ϊ��ͬѧ��ʵ��װ�û�ʹ��õĽ��ƫ�ͣ���ͬѧ��Ϊ���ƫ�͵�ԭ���ǣ�
������̼û����ȫ����ʯ������
������̼û����ȫ����ʯ������
����5����Ҫ����ҡ���ͬѧָ���ļ�ͬѧʵ���е����⣬�ɶԼ�ͬѧ��ʵ��װ�ú�ʵ��ҩƷ�����ʵ��ĸĶ����밴����˳��ѡ����ͼ2��ʾ����������±������ô�д��ĸ�����������������ظ�ʹ�ã��г������ԣ�
| ѡ�õ����� | C C |
D D |
C C |
B B |
A A |
| ���ӵ�ҩƷ ����Ҫ�IJ����� |
װ������������Һ����eͨ����� װ������������Һ����eͨ����� |
��Һ©��װϡ���ᣬ��ƿ��װ��Ʒ��Һ ��Һ©��װϡ���ᣬ��ƿ��װ��Ʒ��Һ |
Ũ���� Ũ���� |
��ʯ�ң���Ӧǰ��ֱ�������� ��ʯ�ң���Ӧǰ��ֱ�������� |
��ʯ�� ��ʯ�� |
��������1�����ݳ���������ɣ�A�Ƿ�Һ©����B����ƿ��
��2����ʵ��ǰ��Ҫ���װ�������ԣ�
��3�����ݼ�ʯ�ҵ�����������������̼�����ʵ���������̼��Ƶ����ʵ����͵��ڶ�����̼�����ʵ����������̼��Ƶ�����������
��4���Ӷ�����̼�仯��ȫ����ʯ�����տ��ǣ�
��5�����ݽ�װ�������ɵĶ�����̼���ÿ������ߣ���֤���ɵĶ�����̼��ȫ����ʯ������ԭ�����ѡ��
��2����ʵ��ǰ��Ҫ���װ�������ԣ�
��3�����ݼ�ʯ�ҵ�����������������̼�����ʵ���������̼��Ƶ����ʵ����͵��ڶ�����̼�����ʵ����������̼��Ƶ�����������
��4���Ӷ�����̼�仯��ȫ����ʯ�����տ��ǣ�
��5�����ݽ�װ�������ɵĶ�����̼���ÿ������ߣ���֤���ɵĶ�����̼��ȫ����ʯ������ԭ�����ѡ��
����⣺��1������A��B�����Ʒֱ��ǣ���Һ©������ƿ���ʴ�Ϊ����Һ©������ƿ��
��2����ʵ�鿪ʼǰ��Ҫ���װ�õ������ԣ������Ӻ�װ�ã�
�ʴ�Ϊ�����װ�������ԣ����Ӻ�װ�ã�
��3������̼��ƺ����ᷴӦ��ϵʽCO2��Na2CO3��������̼�����ʵ�������̼��Ƶ����ʵ�����������̼�������ǣ���c-b��g�����ʵ����ǣ�
mol��̼��Ƶ����������ǣ�
=
��
�ʴ�Ϊ��
��
��4��̼������ɵĶ�����̼�����ִ�����Bװ���У�������ȫ����ʯ�����գ�
�ʴ�Ϊ��������̼û����ȫ����ʯ�����գ�
��5�����մ������ҵ�˳��װ�÷ֱ���C��D��C��B��A��C��װ������������Һ����ͨ��Ŀ����еĶ�����̼��������֤װ�õ����ɵĶ�����̼��ȫ����ʯ�����գ�
Dװ���з�Һ©����ѡ��ϡ���ᣬ����ӷ���C��װ��Ũ���ᣬ���ڸ��������̼������ˮ�ָ��ţ�Bװ����װ�м�ʯ�ң����ڲⶨ���ɵĶ�����̼������Dװ��װ�м�ʯ�ң��������տ����еĶ�����̼��ˮ������Ӱ��B��������
�ʴ�Ϊ��
��2����ʵ�鿪ʼǰ��Ҫ���װ�õ������ԣ������Ӻ�װ�ã�
�ʴ�Ϊ�����װ�������ԣ����Ӻ�װ�ã�
��3������̼��ƺ����ᷴӦ��ϵʽCO2��Na2CO3��������̼�����ʵ�������̼��Ƶ����ʵ�����������̼�������ǣ���c-b��g�����ʵ����ǣ�
| c-b |
| 44 |
| ||
| a |
| 53(b-a) |
| 22a |
�ʴ�Ϊ��
| 53(b-a) |
| 22a |
��4��̼������ɵĶ�����̼�����ִ�����Bװ���У�������ȫ����ʯ�����գ�
�ʴ�Ϊ��������̼û����ȫ����ʯ�����գ�
��5�����մ������ҵ�˳��װ�÷ֱ���C��D��C��B��A��C��װ������������Һ����ͨ��Ŀ����еĶ�����̼��������֤װ�õ����ɵĶ�����̼��ȫ����ʯ�����գ�
Dװ���з�Һ©����ѡ��ϡ���ᣬ����ӷ���C��װ��Ũ���ᣬ���ڸ��������̼������ˮ�ָ��ţ�Bװ����װ�м�ʯ�ң����ڲⶨ���ɵĶ�����̼������Dװ��װ�м�ʯ�ң��������տ����еĶ�����̼��ˮ������Ӱ��B��������
�ʴ�Ϊ��
| ѡ�õ����� | C | D | C | B | A |
| ���ӵ�ҩƷ ����Ҫ�IJ����� |
װ������������Һ����eͨ����� | ��Һ©��װϡ���ᣬ��ƿ��װ��Ʒ��Һ | Ũ���� | ��ʯ�ң���Ӧǰ��ֱ�������� | ��ʯ�� |
���������������͵Ĺؼ��Ƿ���ʵ��Ŀ�ĺ�̽������������ʵ����ͨ�����ɵĶ�����̼���������ⶨ̼������Ʒ�ijɷֺ���������ʵ��ijɰ������ռ��Ķ�����̼�������Ƿ�ȷ��

��ϰ��ϵ�д�
�����Ŀ
 NaCl���ʣ�Ϊ��
NaCl���ʣ�Ϊ��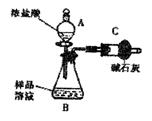
 ���ظ�ʹ�ã��г������ԣ�
���ظ�ʹ�ã��г������ԣ�