��Ŀ����
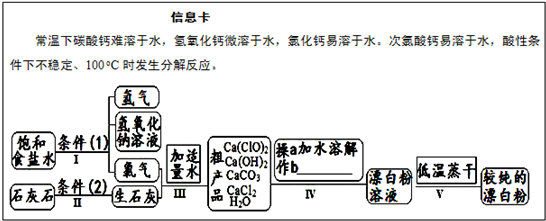
Ư����һ�ֳ���ɱ����������������Ч�ɷ�Ϊ������ƣ���ͼΪ��ȡƯ�۵Ĺ�ҵ���̼�ͼ��

��1������ I �õ�Ũ��Ϊ80g?L-1����������Һ�������ʵ���Ũ����______ mol?L-1��
��2��д������ I������Ӧ�Ļ�ѧ��Ӧ����ʽ������˫���ű�ʾ�����ת�Ƶķ������Ŀ��______������ II�ķ�Ӧ������2����______��
��3������ III�з���������Ӧ��д������һ����������ԭ��Ӧ�Ļ�ѧ��Ӧ����______��
��4������ IV�в���b�ǣ�______����д���ƣ�
��5���û�ѧ����ʽ˵���ϴ��Ĵ�����Ʒ�ĩ�����ܷⱣ���ԭ��______��

��1������ I �õ�Ũ��Ϊ80g?L-1����������Һ�������ʵ���Ũ����______ mol?L-1��
��2��д������ I������Ӧ�Ļ�ѧ��Ӧ����ʽ������˫���ű�ʾ�����ת�Ƶķ������Ŀ��______������ II�ķ�Ӧ������2����______��
��3������ III�з���������Ӧ��д������һ����������ԭ��Ӧ�Ļ�ѧ��Ӧ����______��
��4������ IV�в���b�ǣ�______����д���ƣ�
��5���û�ѧ����ʽ˵���ϴ��Ĵ�����Ʒ�ĩ�����ܷⱣ���ԭ��______��
��1�����ʵ���Ũ��c=
=
=
=2mol/L���ʴ�Ϊ��2mol/L��
��2����ⱥ��ʳ��ˮ�ķ�ӦΪ����2NaCl+2H2O
2NaOH+Cl2��+H2�������ϼ�����ֵ=���ϼ۽���ֵ=ת�Ƶ�����=2������ת�����Ϊ��
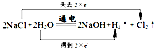
��̼����ڸ����»�ֽ⣬�ʴ�Ϊ��
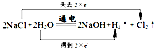
�����£�
��3����Ԫ�ػ��ϼ۱仯�ķ�ӦΪ������ԭ��Ӧ������III�з���������Ӧ��һ�����������������Ʒ�Ӧ�����Ȼ��ơ�ˮ�Լ�������Ƶķ�Ӧ����2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O������������ԭ��Ӧ��һ���Ǵ�����ƺ�ˮ�Լ�������̼��Ӧ����̼��ƺʹ�����ķ�Ӧ����Ca��ClO��2+CO2+H2O�TCaCO3+2HClO�����ڸ��ֽⷴӦ��һ������������ԭ��Ӧ��
�ʴ�Ϊ��2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O��
��4������IV�м�ˮ�ܽ����˿���ʵ�ֹ����������Һ��ķ��룬�ʴ�Ϊ�����ˣ�
��5���ϴ��Ĵ�����Ʒ�ĩ�����ܷⱣ�棬��Ϊ������ԺͿ����е�ˮ�Լ�������̼��Ӧ�����ʣ���Ca��ClO��2+CO2+H2O�TCaCO3+2HClO���ʴ�Ϊ��Ca��ClO��2+CO2+H2O�TCaCO3+2HClO��
| n |
| V |
| ||
| V |
| ||
| 1L |
��2����ⱥ��ʳ��ˮ�ķ�ӦΪ����2NaCl+2H2O
| ||
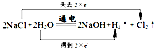
��̼����ڸ����»�ֽ⣬�ʴ�Ϊ��
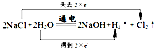
�����£�
��3����Ԫ�ػ��ϼ۱仯�ķ�ӦΪ������ԭ��Ӧ������III�з���������Ӧ��һ�����������������Ʒ�Ӧ�����Ȼ��ơ�ˮ�Լ�������Ƶķ�Ӧ����2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O������������ԭ��Ӧ��һ���Ǵ�����ƺ�ˮ�Լ�������̼��Ӧ����̼��ƺʹ�����ķ�Ӧ����Ca��ClO��2+CO2+H2O�TCaCO3+2HClO�����ڸ��ֽⷴӦ��һ������������ԭ��Ӧ��
�ʴ�Ϊ��2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O��
��4������IV�м�ˮ�ܽ����˿���ʵ�ֹ����������Һ��ķ��룬�ʴ�Ϊ�����ˣ�
��5���ϴ��Ĵ�����Ʒ�ĩ�����ܷⱣ�棬��Ϊ������ԺͿ����е�ˮ�Լ�������̼��Ӧ�����ʣ���Ca��ClO��2+CO2+H2O�TCaCO3+2HClO���ʴ�Ϊ��Ca��ClO��2+CO2+H2O�TCaCO3+2HClO��

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д� ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ
Ư����һ�ֳ���ɱ����������������Ч�ɷ�Ϊ������ƣ���ͼΪ��ȡƯ�۵Ĺ�ҵ���̼�ͼ��
| ��Ϣ�� ������̼���������ˮ��������������ˮ���Ȼ���������ˮ���������������ˮ�����������²��ȶ���100��Cʱ�����ֽⷴӦ�� 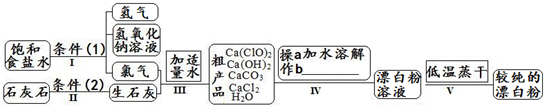 ��1������I �õ�Ũ��Ϊ80g?L-1����������Һ�������ʵ���Ũ���� 2 2 mol?L-1����2��д������I������Ӧ�Ļ�ѧ��Ӧ����ʽ������˫���ű�ʾ�����ת�Ƶķ������Ŀ��   ���� ���� ����3������III�з���������Ӧ��д�����е�������ԭ��Ӧ�Ļ�ѧ��Ӧ���� 2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O 2Cl2+2Ca��OH��2�TCa��ClO��2+CaCl2+2H2O ��������������Cl2 Cl2 ����ԭ����Cl2 Cl2 ����д��ѧʽ����4������IV�в���b�ǣ� ���� ���� ����д���ƣ���5������V���е������ɵ�ԭ���ǣ� Ca��ClO��2��100��Cʱ�����ֽⷴӦ Ca��ClO��2��100��Cʱ�����ֽⷴӦ ����6���û�ѧ����ʽ˵���ϴ��Ĵ�����Ʒ�ĩ�����ܷⱣ���ԭ�� Ca��ClO��2+CO2+H2O�TCaCO3+2HClO Ca��ClO��2+CO2+H2O�TCaCO3+2HClO ����7����ˮ�ʹ�����ƶ�����Ư�ס��������ã�����Ϊ���Ƕ��ܲ���ͬһ�����ʣ�д��������ˮ��Ӧ���������ʵĻ�ѧ����ʽ Cl2+H2O  HCl+HClO HCl+HClOCl2+H2O �� HCl+HClO HCl+HClO��8�������������´�����Ƶ������Ա�����ǿ���ܽ��������������嵥�ʣ�����������е���Ԫ����ԭΪ��һ�۵������ӣ���Ԫ����ת��Ϊˮ����д��������ƺ�ϡ����Ļ�������廯�Ʒ�Ӧ�Ļ�ѧ����ʽ�� Ca��ClO��2+4HCl+4NaBr=CaCl2+4NaCl+2Br2+2H2O Ca��ClO��2+4HCl+4NaBr=CaCl2+4NaCl+2Br2+2H2O ��
Ư����һ�ֳ���ɱ����������������Ч�ɷ�Ϊ������ƣ���ͼΪ��ȡƯ�۵Ĺ�ҵ���̼�ͼ��
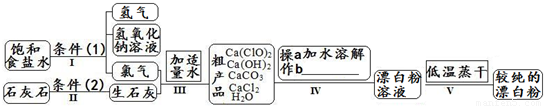 ��1������I �õ�Ũ��Ϊ80g?L-1����������Һ�������ʵ���Ũ����______ mol?L-1�� ��2��д������I������Ӧ�Ļ�ѧ��Ӧ����ʽ������˫���ű�ʾ�����ת�Ƶķ������Ŀ��______������II�ķ�Ӧ������2����______�� ��3������III�з���������Ӧ��д�����е�������ԭ��Ӧ�Ļ�ѧ��Ӧ����______��������������______����ԭ����______����д��ѧʽ�� ��4������IV�в���b�ǣ�______����д���ƣ� ��5������V���е������ɵ�ԭ���ǣ�______�� ��6���û�ѧ����ʽ˵���ϴ��Ĵ�����Ʒ�ĩ�����ܷⱣ���ԭ��______�� ��7����ˮ�ʹ�����ƶ�����Ư�ס��������ã�����Ϊ���Ƕ��ܲ���ͬһ�����ʣ�д��������ˮ��Ӧ���������ʵĻ�ѧ����ʽ______�� ��8�������������´�����Ƶ������Ա�����ǿ���ܽ��������������嵥�ʣ�����������е���Ԫ����ԭΪ��һ�۵������ӣ���Ԫ����ת��Ϊˮ����д��������ƺ�ϡ����Ļ�������廯�Ʒ�Ӧ�Ļ�ѧ����ʽ��______�� |