��Ŀ����
����������һ�ֹ㷺ʹ�õĻ�ѧ�Լ���ij����С��ͨ������ʵ��ⶨij������Ba(OH)2��nH2O�ĺ�����?��1����ȡ3.50 g������������ˮ���100 mL��Һ������ȡ��10.0 mL��Һ����ƿ�У���2��ָʾ������0.100 mol��L-1������ζ����յ㣬�����ı�Һ20.0 mL(���ʲ����ᷴӦ�������������������������ʵ�����?
��2����ȡ5.25 g����������ʧȥȫ���ᾧˮ�����ʲ��ֽ⣩���Ƶ�����Ϊ3.09 g,��Ba(OH)2��nH2O�е�nֵ��?
��3��������Ba(OH)2��nH2O����������Ϊ���٣�??????
�������������ɷ���ʽ���3.5 g��Ʒ��Ba(OH)2�����ʵ����������ݼ���ʧˮ�����������ˮ�����ʵ�������nֵҲ����á�ֻҪ�������������Ϳ������Ʒ�Ĵ��ȡ�?
�𰸣���1��n��Ba(OH)2��=![]() n(HCl)��
n(HCl)��![]() =
=![]() ��0.100 mol��L-1��20��10-3 L��10=0.01 mol��?
��0.100 mol��L-1��20��10-3 L��10=0.01 mol��?
��2��5.25 g������Ba(OH)2�����ʵ���Ϊ![]() ��0.01 mol=0.015 mol,����H2O�����ʵ���Ϊ
��0.01 mol=0.015 mol,����H2O�����ʵ���Ϊ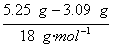 =0.12 mol��n��ֵΪ
=0.12 mol��n��ֵΪ![]() =8��?
=8��?
��3��3.50 g������Ba(OH)2��8H2O������Ϊ0.01 mol��315 g��mol-1=3.15 g���������У�Ba(OH)2��nH2O����������Ϊ![]() ��100%=90%��
��100%=90%��

��ϰ��ϵ�д�
�����Ŀ