��Ŀ����
�ơ�þ���������Ƚ������ճ������ҵ������������ҵ�����Ź㷺����;��
��1��4.8gAl�ڿ����з���һ��ʱ��֮��������Ϊ5.28g����δ�����仯����������Ϊ g��
��2����10mL 0.2mol/L���Ȼ�����Һ����μ���δ֪Ũ�ȵ�����������Һ����õμ�10mL��μ�30mLʱ���ó���һ���࣮������������Һ�����ʵ���Ũ�ȣ�
��3����þ�����Ͻ�6.3g�������뵽ijŨ�ȵ������У���������µ�����8.96L�����跴Ӧ�����л�ԭ������NO��NO2�Ļ�����壩����������Һ�м��백ˮ�������ﵽ�����������������õ�������ԭ�Ͻ����������10.2g�����㻹ԭ������NO��NO2�����ʵ���֮�ȣ�
��4������һ�����ȼ������ۺ���������ĩ�Ļ����ڸ�����ʹ֮��ַ�Ӧ������Ӧ��Ĺ����Ϊ���ȷݣ���������ʵ�飨����pHʱ�ٶ���Һ���û�б仯������������һ�ݹ����м���50mL 3.0mol/L��NaOH��Һ������ʹ���ַ�Ӧ����ˣ������Һ��c��OH-��Ϊ1.0mol/L��������һ�ݹ����м���100mL 4.0mol/L��HCl��Һ��ʹ����ȫ���ܽ⣬��÷�Ӧ��������Һ��ֻ��H+��Fe2+��Al3+������������c��H+��Ϊ0.2mol/L������������ȼ���������������������������
��1��4.8gAl�ڿ����з���һ��ʱ��֮��������Ϊ5.28g����δ�����仯����������Ϊ
��2����10mL 0.2mol/L���Ȼ�����Һ����μ���δ֪Ũ�ȵ�����������Һ����õμ�10mL��μ�30mLʱ���ó���һ���࣮������������Һ�����ʵ���Ũ�ȣ�
��3����þ�����Ͻ�6.3g�������뵽ijŨ�ȵ������У���������µ�����8.96L�����跴Ӧ�����л�ԭ������NO��NO2�Ļ�����壩����������Һ�м��백ˮ�������ﵽ�����������������õ�������ԭ�Ͻ����������10.2g�����㻹ԭ������NO��NO2�����ʵ���֮�ȣ�
��4������һ�����ȼ������ۺ���������ĩ�Ļ����ڸ�����ʹ֮��ַ�Ӧ������Ӧ��Ĺ����Ϊ���ȷݣ���������ʵ�飨����pHʱ�ٶ���Һ���û�б仯������������һ�ݹ����м���50mL 3.0mol/L��NaOH��Һ������ʹ���ַ�Ӧ����ˣ������Һ��c��OH-��Ϊ1.0mol/L��������һ�ݹ����м���100mL 4.0mol/L��HCl��Һ��ʹ����ȫ���ܽ⣬��÷�Ӧ��������Һ��ֻ��H+��Fe2+��Al3+������������c��H+��Ϊ0.2mol/L������������ȼ���������������������������
��������1���������������Ĺ�ϵ���ò��������㣻
��2�����Ȼ�����Һ����μ�������������Һʱ���������ӷ�Ӧ����ʽΪ��Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+H2O����������֮��Ĺ�ϵʽ���㣻
��3�����ݽ�����������ת��Ϊ����ʱ�������ӵ���������þ���������ʵ������ٸ���������ԭ��Ӧ�е�ʧ�������Ƽ���һ�������Ͷ�����̼�����ʵ���֮�ȣ�
��4�����������֪���������ƹ��������ݷ�Ӧ���������Ƽ���ƫ�����Ƶ����ʵ������ٸ�����ԭ���غ��������������
����Һ�����ԣ�˵����Һ��������������ݢ�֪�������ӵ����ʵ������ٸ��ݵ���غ���������ӵ����ʵ���������������ʽ������������������
��2�����Ȼ�����Һ����μ�������������Һʱ���������ӷ�Ӧ����ʽΪ��Al3++3OH-=Al��OH��3����Al��OH��3+OH-=AlO2-+H2O����������֮��Ĺ�ϵʽ���㣻
��3�����ݽ�����������ת��Ϊ����ʱ�������ӵ���������þ���������ʵ������ٸ���������ԭ��Ӧ�е�ʧ�������Ƽ���һ�������Ͷ�����̼�����ʵ���֮�ȣ�
��4�����������֪���������ƹ��������ݷ�Ӧ���������Ƽ���ƫ�����Ƶ����ʵ������ٸ�����ԭ���غ��������������
����Һ�����ԣ�˵����Һ��������������ݢ�֪�������ӵ����ʵ������ٸ��ݵ���غ���������ӵ����ʵ���������������ʽ������������������
����⣺��1�����������ķ�Ӧ����ʽΪ4Al+3O2=2Al2O3�����ݷ���ʽ֪���������ӵ���������������������
��δ�μӷ�Ӧ����������Ϊag����μӷ�Ӧ����������Ϊ��4.8-a��g
4Al+3O2=2Al2O3 ������������
108 96
��4.8-a��g ��5.28-4.8��g
108��96=��4.8-a��g����5.28-4.8��g
9��8=��4.8-a����0.48��
a=4.26g��
�ʴ�Ϊ��4.26g��
��2��n��AlCl3��=0.2mol/L��0.01L=0.002mol��
���������������ʵ���Ũ��Ϊx��
2AlCl3+3Ba��OH��2=2Al��OH��3��+3BaCl2
2 3 2
0.01xmol
mol
������ԭ���غ�֪������ȫת��Ϊ������������ʱ��n��[Al��OH��3]=n��AlCl3��=0.002mol��
����2AlCl3+3Ba��OH��2=2Al��OH��3��+3BaCl2֪����ȫ����ʱ��Ҫn[Ba��OH��2]=
��3=0.003mol��
��������֪��������������Ӧ���������������ʵ���=��0.03x-0.003��mol��
����2Al��OH��3+Ba��OH��2=Ba��AlO2��2+4H2O��֪������ƫ���ᱵ��Ҫn[Al��OH��3]=2n[Ba��OH��2]=2��0.03x-0.003��mol��
������ԭ���غ��ʣ��n[Al��OH��3]=0.002mol-2��0.03x-0.003��mol��
��õμ�10mL��μ�30mL���ó���ͬ���࣬����
mol=0.002mol-2��0.03x-0.003��mol��
x=0.12mol/L��
���������������ʵ���Ũ��Ϊ0.12 mol/L��
��3��þ�������ת��Ϊ����������þ������������������������������������ӵ������������������ӵ����ʵ���=
=0.6mol��
��þ�����ʵ���Ϊxmol���������ʵ���Ϊymol�����ݽ������ʵ�������ת��Ϊ�������ӵ������з��̵�
��
���
��
þ���������ᷴӦ���ɵ�������ʱת�Ƶ�����ȣ�������������ʵ���=
=0.4mol��ÿ������������һ�����������ж�ֻ��һ����ԭ�ӣ�
��һ���������������������ʵ����ֱ�Ϊn��NO����n��NO2����
��
���
��
��һ�������Ͷ������������ʵ���֮��Ϊ0.1mol��0.3mol=1��3��
��һ�������Ͷ������������ʵ���֮��Ϊ1��3��
��4�������������ƹ�������Һ�е�������ƫ�����ƺ��������ƣ���Һ��n��NaOH��=1.0mol/L��0.05L=0.05mol��������ԭ���غ��n��NaAlO2��=��3.0-1.0��mol/L��0.05L=0.1mol��������ԭ���غ�֪���������ʵ���Ҳ��0.1mol����m��Al��=0.1mol��27g/mol=2.7g��ÿ��������������ȣ��������ȼ�������������5.4g��
�ڸ���Һ�����ԣ���������������ݢ�֪������Һ�������ӵ����ʵ���Ϊ0.1mol�������ӵ����ʵ���Ũ��=
=1mol/L����Һ�ʵ����ԣ������������������ȣ����������ӵ����ʵ���Ϊn��Fe 2+ �������ݵ���غ��c��H+��+2 c��Fe 2+ ��+3n��Al3+ ��=c��Cl-����
0.2mol/L+2c��Fe 2+��+3��1mol/L=4.0mol/L��
c��Fe 2+��=0.4mol/L��������ԭ���غ����n��Fe2O3��=
n��Fe 2+��=
��0.4mol/L��0.1L=0.02mol��m��Fe2O3��=0.02mol��160g/mol=3.2g��������������������ȣ��������ȼ���������������Ϊ6.4g��
�����ȼ�������������5.4g����������������6.4g��
��δ�μӷ�Ӧ����������Ϊag����μӷ�Ӧ����������Ϊ��4.8-a��g
4Al+3O2=2Al2O3 ������������
108 96
��4.8-a��g ��5.28-4.8��g
108��96=��4.8-a��g����5.28-4.8��g
9��8=��4.8-a����0.48��
a=4.26g��
�ʴ�Ϊ��4.26g��
��2��n��AlCl3��=0.2mol/L��0.01L=0.002mol��
���������������ʵ���Ũ��Ϊx��
2AlCl3+3Ba��OH��2=2Al��OH��3��+3BaCl2
2 3 2
0.01xmol
| 0.01x��2 |
| 3 |
������ԭ���غ�֪������ȫת��Ϊ������������ʱ��n��[Al��OH��3]=n��AlCl3��=0.002mol��
����2AlCl3+3Ba��OH��2=2Al��OH��3��+3BaCl2֪����ȫ����ʱ��Ҫn[Ba��OH��2]=
| 0.002mol |
| 2 |
��������֪��������������Ӧ���������������ʵ���=��0.03x-0.003��mol��
����2Al��OH��3+Ba��OH��2=Ba��AlO2��2+4H2O��֪������ƫ���ᱵ��Ҫn[Al��OH��3]=2n[Ba��OH��2]=2��0.03x-0.003��mol��
������ԭ���غ��ʣ��n[Al��OH��3]=0.002mol-2��0.03x-0.003��mol��
��õμ�10mL��μ�30mL���ó���ͬ���࣬����
| 0.02x |
| 3 |
x=0.12mol/L��
���������������ʵ���Ũ��Ϊ0.12 mol/L��
��3��þ�������ת��Ϊ����������þ������������������������������������ӵ������������������ӵ����ʵ���=
| 10.2g |
| 17g/mol |
��þ�����ʵ���Ϊxmol���������ʵ���Ϊymol�����ݽ������ʵ�������ת��Ϊ�������ӵ������з��̵�
|
���
|
þ���������ᷴӦ���ɵ�������ʱת�Ƶ�����ȣ�������������ʵ���=
| 8.96L |
| 22.4L/mol |
��һ���������������������ʵ����ֱ�Ϊn��NO����n��NO2����
|
���
|
��һ�������Ͷ������������ʵ���֮��Ϊ0.1mol��0.3mol=1��3��
��һ�������Ͷ������������ʵ���֮��Ϊ1��3��
��4�������������ƹ�������Һ�е�������ƫ�����ƺ��������ƣ���Һ��n��NaOH��=1.0mol/L��0.05L=0.05mol��������ԭ���غ��n��NaAlO2��=��3.0-1.0��mol/L��0.05L=0.1mol��������ԭ���غ�֪���������ʵ���Ҳ��0.1mol����m��Al��=0.1mol��27g/mol=2.7g��ÿ��������������ȣ��������ȼ�������������5.4g��
�ڸ���Һ�����ԣ���������������ݢ�֪������Һ�������ӵ����ʵ���Ϊ0.1mol�������ӵ����ʵ���Ũ��=
| 0.1mol |
| 0.1L |
0.2mol/L+2c��Fe 2+��+3��1mol/L=4.0mol/L��
c��Fe 2+��=0.4mol/L��������ԭ���غ����n��Fe2O3��=
| 1 |
| 2 |
| 1 |
| 2 |
�����ȼ�������������5.4g����������������6.4g��
��������������Ϊ���忼�������ʵ������йؼ��㣬��ȷ����֮��ķ�Ӧ���ԭ���غ㡢ת�Ƶ����غ����������ע�⣨4���кܶ�ͬѧ����ֻ����һ�������������������������´���Ϊ�״��㣮

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ
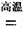 TiCl4+2CO
TiCl4+2CO H����393��5 kJ��mol-1
H����393��5 kJ��mol-1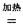 4NaCl+Ti���÷�Ӧ������ˮ��Һ�н��У�һ����ΪTiCl4��ǿ��ˮ������TiO2����һԭ�� �����ʵ���ѧ����ʽ���Ա�Ҫ������˵������
4NaCl+Ti���÷�Ӧ������ˮ��Һ�н��У�һ����ΪTiCl4��ǿ��ˮ������TiO2����һԭ�� �����ʵ���ѧ����ʽ���Ա�Ҫ������˵������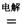 Mg+Cl2 TiCl4+2Mg
Mg+Cl2 TiCl4+2Mg 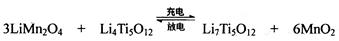 ��ʹ��ʱ�ȳ�磬д������ʽ��������Ӧ ���ŵ�ʱLi+���ƶ����� ��
��ʹ��ʱ�ȳ�磬д������ʽ��������Ӧ ���ŵ�ʱLi+���ƶ����� ��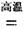 TiCl4+2CO
TiCl4+2CO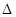 H����393��5 kJ��mol-1
H����393��5 kJ��mol-1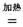 4NaCl+Ti���÷�Ӧ������ˮ��Һ�н��У�һ����ΪTiCl4��ǿ��ˮ������TiO2����һԭ��
�����ʵ���ѧ����ʽ���Ա�Ҫ������˵������
4NaCl+Ti���÷�Ӧ������ˮ��Һ�н��У�һ����ΪTiCl4��ǿ��ˮ������TiO2����һԭ��
�����ʵ���ѧ����ʽ���Ա�Ҫ������˵������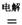 Mg+Cl2
TiCl4+2Mg
Mg+Cl2
TiCl4+2Mg 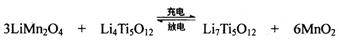 ��ʹ��ʱ�ȳ�磬д������ʽ��������Ӧ ���ŵ�ʱLi+���ƶ�����
��
��ʹ��ʱ�ȳ�磬д������ʽ��������Ӧ ���ŵ�ʱLi+���ƶ�����
��