��Ŀ����
��1����ӡˢ��·ʱ�����Ȼ�����Һ��Ϊ����ʴҺ����ͭ���Ȼ�����Һ��ʴ�ķ���ʽΪ��2FeCl3+Cu=2FeCl2+CuCl2���Ȼ�����ҺҲ��������Ӧ��2FeCl3+Fe=3FeCl2������ʢ���Ȼ�����Һ���ձ���ͬʱ�������ۺ�ͭ�ۣ���Ӧ�������ձ��ײ����ܳ��ֵ������______����ѡ��
A����ͭ����B��������ͭC��������ͭD��������ͭ
���ͭ���Ȼ�����Һ��ʴ�Ļ�ѧ����ʽ��дΪ���ӷ���ʽ��______��
��2��ij�ӵ������м��������������ŷŵĹ�ҵ��ˮ�У������� K+��Ag+��Fe3+��C1-?��OH-��NO3-�������ӣ�����ü׳��ķ�ˮ���Գʼ��ԣ������֪�ҳ���ˮ������������������______��
�⣺��1�����ս�����Ա���֪Fe��Cu���ã�������Fe3+��Ӧ��������ʣ��ʱ��һ��ʣ��ͭ����ͭʣ��ʱ������ʣ������Ҳ������ȫ��Ӧ����FeCl3ʣ��ʱ������ͭ����ȫ��Ӧ��û��ʣ�࣬FeCl3��FeCl2��CuCl2������ˮ���������ʣ�д�������γɣ�ͭ���Ȼ�����Һ��ʴ�Ļ�ѧ����ʽ��дΪ���ӷ���ʽΪ2Fe3++Cu=2Fe2++Cu2+���ʴ�Ϊ��ACD��2Fe3++Cu=2Fe2++Cu2+��
��2���׳��ķ�ˮ���Գʼ��ԣ�����Һ�д��ڴ�����OH-����OH-��Ӧ��Ag+��Fe3+����Ӧ�������ҳ���������Һ������ԭ�׳�һ������������K+���ҳ�����Ag+������Ag+��Ӧ��C1-?���Ӵ����ڼ׳���������Һ������ԭ���ҳ�����NO3-��
�ʴ�Ϊ��Ag+��Fe3+��NO3-��
��������1�����ݽ����Ļ�ԭ��ǿ��������
��2���׳��ķ�ˮ���Գʼ��ԣ�����Һ�д��ڴ�����OH-��������OH-��Ӧ������Ӧ�������ҳ��������Һ�����Ե�ԭ���һ���ƶϣ�
���������⿼�����ӹ������⣬��Ŀ�ѶȲ���ע������֮��ķ�Ӧ�����Լ����ӵ����ʣ�ע�ػ���֪ʶ�Ļ��ۣ�
��2���׳��ķ�ˮ���Գʼ��ԣ�����Һ�д��ڴ�����OH-����OH-��Ӧ��Ag+��Fe3+����Ӧ�������ҳ���������Һ������ԭ�׳�һ������������K+���ҳ�����Ag+������Ag+��Ӧ��C1-?���Ӵ����ڼ׳���������Һ������ԭ���ҳ�����NO3-��
�ʴ�Ϊ��Ag+��Fe3+��NO3-��
��������1�����ݽ����Ļ�ԭ��ǿ��������
��2���׳��ķ�ˮ���Գʼ��ԣ�����Һ�д��ڴ�����OH-��������OH-��Ӧ������Ӧ�������ҳ��������Һ�����Ե�ԭ���һ���ƶϣ�
���������⿼�����ӹ������⣬��Ŀ�ѶȲ���ע������֮��ķ�Ӧ�����Լ����ӵ����ʣ�ע�ػ���֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
 F��g��+D��g����Ӧ���ʺ�ʱ��Ĺ�ϵ��ͼ2��ʾ����ô��t1ʱ���ʷ����ı��ԭ������ǣ���ѡ����ĸ��______����
F��g��+D��g����Ӧ���ʺ�ʱ��Ĺ�ϵ��ͼ2��ʾ����ô��t1ʱ���ʷ����ı��ԭ������ǣ���ѡ����ĸ��______���� 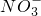
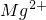
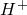
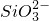
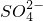
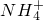
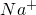
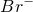
 2NH3��+H2O+SO2��
2NH3��+H2O+SO2�� 2CO2
2CO2 Si+2CO��
Si+2CO��