��Ŀ����
һ�ֺ�����ﮡ��ܵ����͵��Ӳ��ϣ������в����ķ��������ɹۣ������е����Խ�����������ʽ���ڣ�����Co2O3·CoO����ʽ���ڣ������������ĵ����˫�棻﮻��������С�
�ӷ����л��������ܣ�CoO���Ĺ����������£�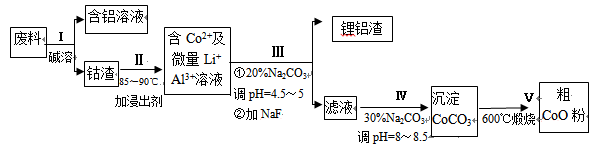
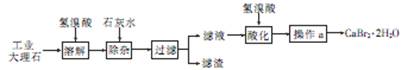
��1������I�в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ ��
��2������II�м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܡ�������ܵĻ�ѧ��Ӧ����ʽΪ��������ֻ��һ������� ����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ�ʹ�ҵ�����в������ᣬ��ӷ�Ӧԭ������������������ܵ���Ҫԭ��_______________��
��3�����̢�õ����������Ҫ�ɷ���LiF��Al(OH)3��̼������Һ�ڲ���Al(OH)3ʱ����Ҫ���ã���д���÷�Ӧ�����ӷ���ʽ________________________��
��4��̼������Һ�ڹ���III��IV����������������ͬ����д���ڹ���IV�����������
____________________________________________________________��
��5����Na2CO3��Һ�д��ڶ������ӣ����и�����Ũ�ȹ�ϵ��ȷ����______������ţ���
| A��c(Na+) = 2c(CO32-) | B��c(Na+) > c(CO32-) > c(HCO3-) |
| C��c(OH-) > c(HCO3-) > c(H+) | D��c(OH-) - c(H+)��c(HCO3-) + 2c(H2CO3) |
��1��2Al+2OH-+2H2O = 2AlO2-+3H2��
��2��4��Co2O3·CoO�� + Na2S2O3 + 11H2SO4 �� 12CoSO4 + Na2SO4 + 11H2O(3��)
Co2O3·CoO�������������Cl2����Ⱦ���������������ɣ�
��3��2Al3++3CO32-+3H2O �� 2Al(OH)3��+3CO2��
��4������pH���ṩCO32-��ʹCo2+����ΪCoCO3
��5��B C D ���д����÷֣����1����1�֣�ȫ�Ե�3�֣�
���������������1�������������Ʒ�Ӧ����ƫ�����ƺ�������ע��÷�Ӧ��ˮ�Ƿ�Ӧ�
��2��Co3O4�������������������������·���������ԭ��Ӧ������������ӡ����������Ӻ�ˮ��������л�ԭ�ԣ��ܱ�Co2O3?CoO���������ж���������
��3����������������̼������ӷ���˫ˮ���������������Ͷ�����̼��
��4��̼������Һ�ڹ��̢�������������̼������ӷ���˫ˮ���������������Ͷ�����̼��̼������Һ�ڹ��̢��е���pH���ṩCO32-��ʹCo2+����ΪCoCO3��
��5��A�����ݵ������жϣ�
B������̼������ӷ���ˮ���Լ�ˮ�ĵ����жϳ�����Ũ�ȵĴ�С��
C������̼������ӷ���ˮ���Լ�ˮ�ĵ����жϳ�����Ũ�ȵĴ�С��
D�����������غ��жϣ�
��6�����ݹ�ϵʽCoCl2?6H2O��CoCl2���CoCl2?6H2O��������Ȼ���ٸ��ݲ��������A���ʵĻ�ѧʽ��
���㣺���ʷ�����ᴿ�����ͻ����������ۺ�Ӧ�ã�����Ũ�ȴ�С�Ƚϡ�

Ϊ̽��ij����ҩX����ɣ���������ʵ�飺
�������ϣ�
�ٿ���ҩX���ܵ���ɿ��Ա�ʾΪ��MgmAln(OH)p(CO3)q(SiO3)r��m��n��p��q��rΪ��0����������
�� ��pH=5.0ʱ������ȫ��
��pH=5.0ʱ������ȫ�� ��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��
��pH=8.8ʱ��ʼ������pH=11.4ʱ������ȫ��
ʵ����̣�
| ���� | ʵ����� | ʵ������ |
| I | ��X�ķ�ĩ�м���������� | ��������A���õ���ɫ��Һ |
| II | ������õ���Һ�еμӰ�ˮ������pH��5~ 6������ | ���ɰ�ɫ����B |
| III | �����B�мӹ���NaOH��Һ | ����ȫ���ܽ� |
| IV | ��II�õ�����Һ�еμ�NaOH��Һ������pH��12 | ���ɰ�ɫ����C |
��1����������A��ʹ����ʯ��ˮ����ǣ�A�Ļ�ѧʽ�� ��
��2��II������B��Ӧ�����ӷ���ʽ�� ��
��3��III��B�ܽⷴӦ�����ӷ���ʽ�� ��
��4������C�Ļ�ѧʽ�� ��
��5��������n(A)�Un(B)�Un(C)=1�U2�U3����X�Ļ�ѧʽ�� ��
���������һ������Ч���ˮ����������ҵ�ϳ�����NaClO��������������Ӧԭ��Ϊ��
���ڼ��������£�����NaClO����Fe(NO3)3�Ƶ�Na2FeO4
3NaClO + 2Fe(NO3)3 + 10NaOH��2Na2FeO4��+ 3NaCl + 6NaNO3 + 5H2O
��Na2FeO4��KOH��Ӧ����K2FeO4��Na2FeO4 + 2KOH��K2FeO4 + 2NaOH
��Ҫ�������������£�
��1���������������ҺpHʱ����pH��ֽ���Բ���pH�Կ��Ƽ������������ʵ������pH��ֽ�ⶨ��ҺpH�IJ����� ��
��2������ͼ�С�ת��������Ӧ�ۣ�����ij�����½��еģ�˵�����¶���Ksp��K2FeO4�� Ksp��Na2FeO4���������������������
��3����Ӧ���¶ȡ�ԭ�ϵ�Ũ�Ⱥ���ȶԸ�����صIJ��ʶ���Ӱ�졣
ͼ1Ϊ��ͬ���¶��£�Fe(NO3)3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죻
ͼ2Ϊһ���¶��£�Fe(NO3)3����Ũ�����ʱ��NaClOŨ�ȶ�K2FeO4�����ʵ�Ӱ�졣
��ҵ����������¶�Ϊ �棬��ʱFe(NO3)3��NaClO������Һ�������Ũ��֮��Ϊ ��
��4��K2FeO4��ˮ��Һ���ס�ˮ�⡱��4FeO42- + 10H2O  4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
| A��H2O | B��CH3COONa������� | C��NH4Cl������� | D��Fe(NO3)3������� |
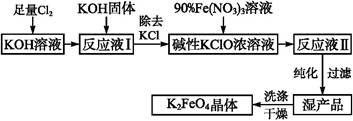
 KCl+KClO+H2O(����:�¶Ƚϵ�)
KCl+KClO+H2O(����:�¶Ƚϵ�) ;��������������������
;��������������������