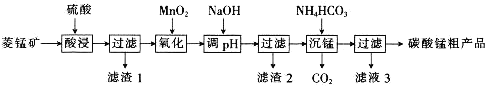
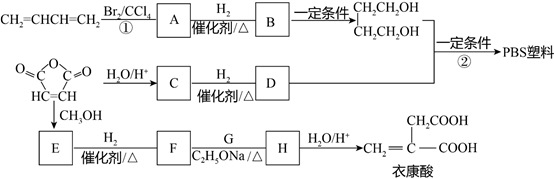
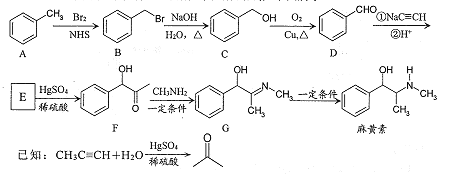
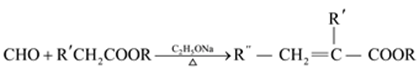��Ŀ����
����Ŀ���Ӻ�ˮ�п�����ȡ�ܶ����õ����ʣ�����Ӻ�ˮ�������õ���±ˮ�п�����ȡ�⡣����̿�������ǹ�ҵ���ķ���֮һ�����������£�

������ʾ����pH=2ʱ��NaNO2��Һֻ�ܽ�I-����ΪI2,ͬʱ����NO��
��I2+5Cl2+6H2O=2HIO3+10HCl��
��5SO32-+2IO3-+2H+=I2+5SO42-+H2O��
����I2�ڼ�����Һ�з�Ӧ����I-��IO3-��
(1)��Ӧ�������ӷ���ʽ_____________��
(2)�������У�����I2�����ԣ��������X��������________________��
(3)��֪����Ӧ����ÿ����3molI2ת��5mol���ӣ������ӷ���ʽ��_______________��
(4)Cl2������KMnO4�ȶ��dz��õ�ǿ�����������ù���������±ˮ�е�I-ȴѡ���˼۸�ϸߵ�NaNO2,ԭ����_______________��
(5)�������У���֪��Ӧ�����˺���Һ���Դ���������I2��I-��IO3-����ֱ������Һ�е�I-��IO3-����ʵ�鷽������������ʵ���пɹ�ѡ����Լ���ϡH2SO4��������Һ��Fe2(SO4)3��Һ��Na2SO3��Һ
A����Һ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���ⵥ�ʴ��ڡ�
B��_______________________��
���𰸡� 2NO2-+2I-+4H+ =I2+2NO��+2H2O ����������ȣ��������ᾧ���������ᾧ���۷֣� 3I2+3CO32-=5I-+IO3-+3CO2������3I2+6CO32-+3H2O=5I-+IO3-+6HCO3-�� ���������Ը�����صȶ��dz��õ�ǿ�����������������I2(���������ƽ��ܰѵ����������ɵⵥ�ʣ���˼�Լ���) ��ˮ��ȡ������Һ���Թ��У����뼸�ε�����Һ���μ�Fe2(SO4)3��Һ������Һ������˵����Һ�к���I��������ˮ����ȡ������Һ���Թ��У����뼸�ε�����Һ���������ữ���μ�Na2SO3��Һ������Һ������˵����Һ�к���IO3��
����������Ӧ���ǵ����ӱ����������������ɵ��ʵ⣬Ȼ�����û���̿�������ʵ⡣�����������õ����������롣������������Ũ̼������Һ���յ��ʵ⣬ת��Ϊ������͵����ӣ���������Һ�ж����ַ���������ԭ��Ӧ���ɵ��ʵ⣬�ݴ˽��
��1����Ӧ��ΪpH=2��Һ�����ԣ�NaNO2��Һ��I������ΪI2��ͬʱ����NO�����䷴Ӧ�����ӷ���ʽΪ��2NO2��+2I��+4H+��I2+2NO��+2H2O��
��2������I2�����������ԣ��������X�ķ�������������ȡ������ᾧ��
��3��������֪��������Ӧ����ÿ����3molI2ת��5mol���ӣ������������ԭ��Ӧ�е�ʧ������Ϊ5����0������Ϊ+5�ۺʹ�0�۽���Ϊ-1�ۣ���������ӷ�Ӧ����ʽ�ǣ�3I2+3CO32��=5I��+IO3��+3CO2����3I2+6CO32��+3H2O=5I��+IO3��+6HCO3����
��4��Cl2������KMnO4�ȶ��dz��õ�ǿ�����������ù���������±ˮ�е�I��ȴѡ���˼۸�ϸߵ�NaNO2��ԭ�������������Ը�����صȶ��dz��õ�ǿ�����������������I2���Ӷ��ò���I2��
��5��������Һ�е�I������ѡ���������������������ĵⵥ�������۱�����������Һ�е�IO3�������û�ԭ�����仹ԭ�����ⵥ�������۱��������ʵ�鷽��Ϊ����ˮ��ȡ������Һ���Թ��У����뼸�ε�����Һ���μ�Fe2(SO4)3��Һ������Һ������˵����Һ�к���I��������ˮ����ȡ������Һ���Թ��У����뼸�ε�����Һ���������ữ���μ�Na2SO3��Һ������Һ������˵����Һ�к���IO3����