��Ŀ����
����Ŀ����ͼ����һ���¶��£��������ˮϡ�͵Ĺ����У���Һ�ĵ����������ˮ���Ĺ�ϵ���ش��������⣺
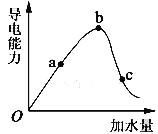
��1��0���㵼������Ϊ0������__________________________________��
��2��a��b��c������Һ��������Ũ����С�����˳��Ϊ________________________��
��3��a��b��c�����У�����ĵ���̶�����һ����_____________��
��4����ʹc����Һ�е�c(CH3COO-)��ߣ������´�ʩ�У���ѡ��__________��
A������ B����ˮ C����CH3COONa���� D���Ӻ�ϡ��NaOH��Һ
��5����ϡ�����У����Ŵ���Ũ�ȵĽ��ͣ�����ʼ�ձ����������Ƶ�����____________��
A��c(H+) B��H+���� C��CH3COOH������ D��c(H+)/c(CH3COOH)
���𰸡� ��Ϊ������δ���룬�������ƶ������� c<a<b c AC BD
�����������⿼��������ʵĵ���ƽ�⣬��1���������Ǵ����ᣬ��������û�����Ӵ��ڣ���ԭ���ǣ���Ϊ������δ���룬�������ƶ������ӣ���2��������������Һ������Ũ���йأ�����Ũ��Խ�࣬��������Խǿ�����������Ũ����С�����˳����c<a<b����3����ˮϡ�ͣ��ٽ�ˮ�ĵ��룬���������̶�������c����4��CH3COOH![]() CH3COO����H����A��������ʵĵ��������ȹ��̣����ȴٽ����룬CH3COO��Ũ������A��ȷ��B����ˮϡ�ͣ���Ȼ�ٽ����룬��CH3COO����Ũ�Ƚ��ͣ���B����C������CH3COONa���壬���ƴ���ĵ��룬��CH3COO��Ũ������C��ȷ��D���Ӻ�ϡ��NaOH��Һ���ٽ�����ĵ��룬��CH3COO��Ũ�Ƚ��ͣ���D����5��A����ϡ���̣�c(H��)���ͣ���A����B����ˮϡ�ͣ��ٽ����룬H���ĸ�������B��ȷ��C����ˮϡ�ͣ��ٽ����룬CH3COOH���������٣���C����D����Ϊ��ͬһ��Һ����Һ�������ͬ���˱�ֵ����n(H��)/n(CH3COOH)����ˮϡ�ͣ��ٽ�����CH3COOH�����ʵ�����С��H�����ʵ���������˴˱�ֵ����D��ȷ��
CH3COO����H����A��������ʵĵ��������ȹ��̣����ȴٽ����룬CH3COO��Ũ������A��ȷ��B����ˮϡ�ͣ���Ȼ�ٽ����룬��CH3COO����Ũ�Ƚ��ͣ���B����C������CH3COONa���壬���ƴ���ĵ��룬��CH3COO��Ũ������C��ȷ��D���Ӻ�ϡ��NaOH��Һ���ٽ�����ĵ��룬��CH3COO��Ũ�Ƚ��ͣ���D����5��A����ϡ���̣�c(H��)���ͣ���A����B����ˮϡ�ͣ��ٽ����룬H���ĸ�������B��ȷ��C����ˮϡ�ͣ��ٽ����룬CH3COOH���������٣���C����D����Ϊ��ͬһ��Һ����Һ�������ͬ���˱�ֵ����n(H��)/n(CH3COOH)����ˮϡ�ͣ��ٽ�����CH3COOH�����ʵ�����С��H�����ʵ���������˴˱�ֵ����D��ȷ��