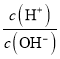��Ŀ����
����Ŀ��������һ�������Դ����������ȡ�봢��������Դ����������о��ص㡣
��֪��CH4(g)��H2O(g)![]() CO(g)��3H2(g) ��H=��206.2 kJ��mol1
CO(g)��3H2(g) ��H=��206.2 kJ��mol1
CH4(g)��CO2(g)![]() 2CO(g)��2H2(g) ��H=��247.4 kJ��mol1
2CO(g)��2H2(g) ��H=��247.4 kJ��mol1
2H2S(g)![]() 2H2(g)��S2(g) ��H=��169.8 kJ��mol1
2H2(g)��S2(g) ��H=��169.8 kJ��mol1
��1���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ____________________________________��
��2��H2S�ȷֽ�����ʱ������Ӧ����ͨ��һ������������ʹ����H2Sȼ�գ���Ŀ����____________��ȼ�����ɵ�SO2��H2S��һ����Ӧ���������ڳ����¾������壬д���÷�Ӧ�Ļ�ѧ����ʽ��
______________________________��
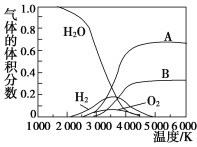
��3��H2O���ȷֽ�Ҳ�ɵõ�H2��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ��ʾ��ͼ��A��B��ʾ������������________________________________________________��
���𰸡���1��CH4(g)��2H2O(g)===CO2(g)��4H2(g) ��H=��165.0 kJ��mol1��2�֣�
��2��ΪH2S�ȷֽⷴӦ�ṩ������2�֣�
2H2S��SO2===2H2O��3S(��4H2S��2SO2===4H2O��3S2) ��2�֣�
��3��H��O(����ԭ�ӡ���ԭ��) ��2�֣�
����������1�����������Ȼ�ѧ����ʽ����2�ټ�ȥ�������Ȼ�ѧ����ʽ���ɵõ���ȷ�𰸡���3���¶���3 000 Kʱ��H2O�ֽ����ԭ�ӡ���ԭ�ӣ���ԭ�ӡ���ԭ�Ӳ��ֽ�ϳ�H2��O2����һ��������ԭ��״̬�����ڻ�������С�5 000 K����ʱ����ԭ�ӡ���ԭ�Ӳ��ٽ�ϳɷ��ӣ���ϵ��ֻ������ԭ�ӡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���ں�����ߺ�DZˮͧ�п��ù���������Ϊ����������ѡ���ʵ��Ļ�ѧ�Լ���ʵ�� ��Ʒ������ͼ�е�ʵ��װ�ý������飬֤���������ƿ�����������
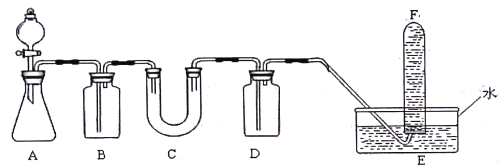
��1��A����ȡCO2��װ�á�д��A�з�����Ӧ�Ļ�ѧ����ʽ��__________________��
��2����д���пո�
���� | �����Լ� | �����Լ���Ŀ�� |
B | ����NaHCO3��Һ | ____________ |
C | _________________ | ____________ |
D | _________________ | ____________ |