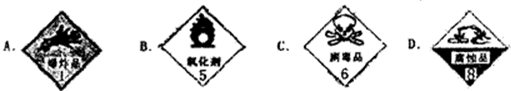
��Ŀ����
��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�
��һ������������ʵ���Ũ��Ϊ
������ʵ�����ø���������240mL0.46mol/L��ϡ���ᣬ��
��1����Ҫ����������Ϊ
��2�����������������ձ���100mL��Ͳ��250mL����ƿ��500mL����ƿ�ݲ�������������ƽ�������룩��10mL��Ͳ�ེͷ�ιܣ�����ʱ������ʹ�õ�������
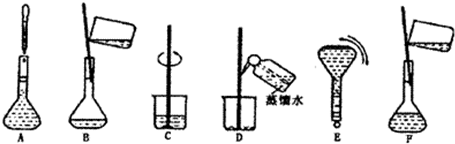
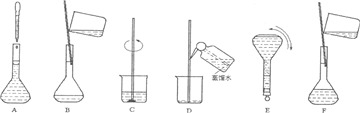
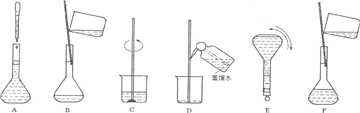
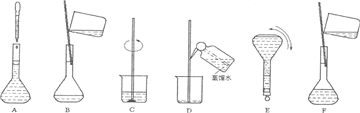
��3�����ƹ������м����ؼ��IJ���Ͳ�������ͼ��ʾ��������ʵ�鲽��A-F��ʵ������Ⱥ��������
��4����ͬѧʵ�����Ƶõ���Ũ��Ϊ0.45mol/L�����ܵ�ԭ����
A����ȡŨH2SO4ʱ���ӿ̶� B������ƿϴ����δ�����ﴦ��
C��û�н�ϴ��Һת������ƿ D������ʱ���ӿ̶ȣ�

��һ������������ʵ���Ũ��Ϊ
18.4mol/L
18.4mol/L
��������ʵ�����ø���������240mL0.46mol/L��ϡ���ᣬ��
��1����Ҫ����������Ϊ
6.3
6.3
mL����2�����������������ձ���100mL��Ͳ��250mL����ƿ��500mL����ƿ�ݲ�������������ƽ�������룩��10mL��Ͳ�ེͷ�ιܣ�����ʱ������ʹ�õ�������
�٢ۢݢߢ�
�٢ۢݢߢ�
������ţ�����3�����ƹ������м����ؼ��IJ���Ͳ�������ͼ��ʾ��������ʵ�鲽��A-F��ʵ������Ⱥ��������
CBDFAE
CBDFAE
��
��4����ͬѧʵ�����Ƶõ���Ũ��Ϊ0.45mol/L�����ܵ�ԭ����
CD
CD
A����ȡŨH2SO4ʱ���ӿ̶� B������ƿϴ����δ�����ﴦ��
C��û�н�ϴ��Һת������ƿ D������ʱ���ӿ̶ȣ�
��������һ������c=
����ŨH2SO4�����ʵ���Ũ�ȣ�
��������1��������Һϡ��ǰ�����ʵ��������������Ũ����������
��2������������Һ�IJ�������ѡ������������
��3����������Һ��ʵ��������̽���ʵ�鲽������
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
�����жϣ�
| 100�Ѧ� |
| M |
��������1��������Һϡ��ǰ�����ʵ��������������Ũ����������
��2������������Һ�IJ�������ѡ������������
��3����������Һ��ʵ��������̽���ʵ�鲽������
��4���������������ʵ����ʵ��������Һ�������Ӱ�죬����c=
| n |
| v |
����⣺��һ���������Լ�ƿ��ǩ�ϵ����ݿ�֪����Ũ�����ܶ�Ϊ1.84g/ml����������Ϊ98%��
����ŨH2SO4�����ʵ���Ũ��c=
mol/L=18.4mol/L��
�ʴ�Ϊ��18.4mol/L��
��������1������ƿû��240ml���ѡ��������������ƿ����Ӧ��250ml������ƿ��
����ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=250mL��0.46mol/L����ã�x��6.3��
�ʴ�Ϊ��6.3��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������10mL��Ͳ��ȡ���õ���ͷ�ιܣ������ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���Ա����õ�����Ϊ�����ձ�����250mL����ƿ���ݲ���������10mL��Ͳ���ེͷ�ιܣ�
�ʴ�Ϊ���٢ۢݢߢ�
��3���ɣ�2���в��������֪��ʵ������Ⱥ��������CBDFAE��
�ʴ�Ϊ��CBDFAE��
��4����ͬѧʵ�����Ƶõ���Ũ��Ϊ0.45mol/L��������ҺŨ��ƫ�ͣ�
A����ȡŨH2SO4ʱ���ӿ̶ȣ���ȡ��Ũ������������������ҺŨ��ƫ�ߣ�
B�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
C��û�н�ϴ��Һת������ƿ��ת�Ƶ�����ƿ��������������ʵ�����С��������ҺŨ��ƫ�ͣ�
D������ʱ���ӿ̶ȣ�ʹ��Һ�����ƫ��������ҺŨ��ƫ�ͣ�
��ѡ��CD��
����ŨH2SO4�����ʵ���Ũ��c=
| 1000��1.84��98% |
| 98 |
�ʴ�Ϊ��18.4mol/L��
��������1������ƿû��240ml���ѡ��������������ƿ����Ӧ��250ml������ƿ��
����ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=250mL��0.46mol/L����ã�x��6.3��
�ʴ�Ϊ��6.3��
��2�����������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�������10mL��Ͳ��ȡ���õ���ͷ�ιܣ������ձ���ϡ�ͣ��ò��������裬��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
���Ա����õ�����Ϊ�����ձ�����250mL����ƿ���ݲ���������10mL��Ͳ���ེͷ�ιܣ�
�ʴ�Ϊ���٢ۢݢߢ�
��3���ɣ�2���в��������֪��ʵ������Ⱥ��������CBDFAE��
�ʴ�Ϊ��CBDFAE��
��4����ͬѧʵ�����Ƶõ���Ũ��Ϊ0.45mol/L��������ҺŨ��ƫ�ͣ�
A����ȡŨH2SO4ʱ���ӿ̶ȣ���ȡ��Ũ������������������ҺŨ��ƫ�ߣ�
B�������Ҫ���ݣ�����ƿ�����������������ˮ������ҺŨ����Ӱ�죻
C��û�н�ϴ��Һת������ƿ��ת�Ƶ�����ƿ��������������ʵ�����С��������ҺŨ��ƫ�ͣ�
D������ʱ���ӿ̶ȣ�ʹ��Һ�����ƫ��������ҺŨ��ƫ�ͣ�
��ѡ��CD��
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ�ע���c=
��������ԭ����ע�����˳������������ƿ����Һ�������ȷ��BFλ�ã�
| n |
| v |

��ϰ��ϵ�д�
�����Ŀ