��Ŀ����
��9�֣���ҵ�ϴӺ�ˮ����ȡ��ķ������£�
�ٽ������Ƶ�ˮ����Ũ���ĺ�ˮ����������ữ��
�����ữ�ĺ�ˮ��ͨ������������ʹ������ת��Ϊ�嵥�ʣ�
����������Һ��ͨ�������ˮ���������嵥�ʴ���ʢ�ж�������ˮ��Һ����������ת���������������������ͨ��������������
�������Ȼ�̼��ȡ�������е��嵥�ʡ�
������������⣺
��1����ʵ����������ˮ�Ƶ�ˮʱ�����õ��������˾ƾ��ơ���ƿ��ţ�ǹܡ������ܡ�ʯ��������Ҫ�ļг�����������Ҫ�IJ��������� ������ʱ���Ƭ��������____________________��
��2��������з�Ӧ�����ӷ���ʽΪ
��3������۵�Ŀ����ʹ�嵥�ʸ�������д���嵥�����������ˮ��Һ��Ӧ�Ļ�ѧ����ʽ ����Ӧ��_________��д��ѧʽ������������1mol�������ڷ�Ӧ�еõ� mol���ӡ�
��4�������ܴ������Ȼ�̼����ȡ����Լ��� ������ţ�
�ٽ������Ƶ�ˮ����Ũ���ĺ�ˮ����������ữ��
�����ữ�ĺ�ˮ��ͨ������������ʹ������ת��Ϊ�嵥�ʣ�
����������Һ��ͨ�������ˮ���������嵥�ʴ���ʢ�ж�������ˮ��Һ����������ת���������������������ͨ��������������
�������Ȼ�̼��ȡ�������е��嵥�ʡ�
������������⣺
��1����ʵ����������ˮ�Ƶ�ˮʱ�����õ��������˾ƾ��ơ���ƿ��ţ�ǹܡ������ܡ�ʯ��������Ҫ�ļг�����������Ҫ�IJ��������� ������ʱ���Ƭ��������____________________��
��2��������з�Ӧ�����ӷ���ʽΪ
��3������۵�Ŀ����ʹ�嵥�ʸ�������д���嵥�����������ˮ��Һ��Ӧ�Ļ�ѧ����ʽ ����Ӧ��_________��д��ѧʽ������������1mol�������ڷ�Ӧ�еõ� mol���ӡ�
��4�������ܴ������Ȼ�̼����ȡ����Լ��� ������ţ�
| A������ | B���ƾ� | C������ | D���� |
��9�֣���1��������ƿ��2�֣�����ֹ���� ��2�֣�
��2��C12+2Br���� Br2+2C1�� ��2�֣�
��3��SO2 + Br2 + 2H2O �� 2HBr + H2SO4 ��2�֣��� Br2��2�֣� 2 ��2�֣�
��4��D ��2�֣�
��2��C12+2Br���� Br2+2C1�� ��2�֣�
��3��SO2 + Br2 + 2H2O �� 2HBr + H2SO4 ��2�֣��� Br2��2�֣� 2 ��2�֣�
��4��D ��2�֣�
��1����������ʵ�顣��������ʵ��ԭ����֪����ȱ�ٵIJ���������������ƿ����Ϊ�ڼ���ʱ����Һ����ҵ��������������Ƭ�������Ƿ�ֹ���С�
��2���������������������ģ��ܰ��������������ɵ����壬����ʽΪC12+2Br���� Br2+2C1����
��3��������Ҳ���������ԣ��ܰ�SO2�����������ᣬ����ʽΪSO2 + Br2 + 2H2O �� 2HBr + H2SO4����Ԫ�صĻ��ϼ���0�۽��͵���1�ۣ�����1mol������õ�2mol���ӡ�
��4��ABC���Ǻ�ˮ���ܵģ�����������ȡ������������ˮ���ҵ����������ڱ��У����Դ�ѡD��
��2���������������������ģ��ܰ��������������ɵ����壬����ʽΪC12+2Br���� Br2+2C1����
��3��������Ҳ���������ԣ��ܰ�SO2�����������ᣬ����ʽΪSO2 + Br2 + 2H2O �� 2HBr + H2SO4����Ԫ�صĻ��ϼ���0�۽��͵���1�ۣ�����1mol������õ�2mol���ӡ�
��4��ABC���Ǻ�ˮ���ܵģ�����������ȡ������������ˮ���ҵ����������ڱ��У����Դ�ѡD��

��ϰ��ϵ�д�
 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ

 ��
�� ��
��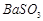 ��
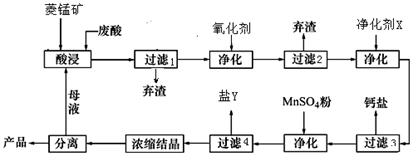
�� ��]��ij��������Ҫ����
��]��ij��������Ҫ����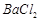 ��
��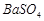 �������ñ�����ȡ
�������ñ�����ȡ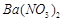 ���䲿�ֹ����������£�����֪����1��FeO2-����ˮ�������Fe(OH)3 (2)Fe3+��pH��3.7ʱ����ˮ�⼴������ȫ��
���䲿�ֹ����������£�����֪����1��FeO2-����ˮ�������Fe(OH)3 (2)Fe3+��pH��3.7ʱ����ˮ�⼴������ȫ��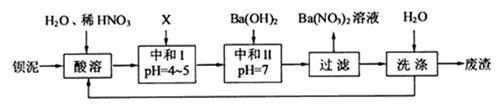
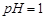 ��
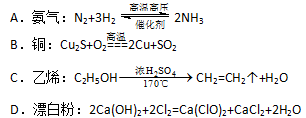
��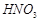 �ķ�Ӧ��ѧ����ʽΪ��
�ķ�Ӧ��ѧ����ʽΪ�� ��������ұ��ͭ����ȸʯ�뽹̿��ϼ���ʱ��������ͭ��������̼��ˮ������˵������ȷ����
��������ұ��ͭ����ȸʯ�뽹̿��ϼ���ʱ��������ͭ��������̼��ˮ������˵������ȷ���� ��������̿��Ӧת��2
��������̿��Ӧת��2 ����
���� ��Ӧ
��Ӧ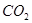 ���������״���������ɲ����Ʒ��
���������״���������ɲ����Ʒ�� �������������ٶ������ɷֲ����ᷴӦ��
�������������ٶ������ɷֲ����ᷴӦ��