��Ŀ����
Ϊ�˷��λ�����Ⱦ����β�������ۺ����ã�ij���᳧�ð�ˮ����β���е�SO2����������Һ�м���Ũ���ᣬ����ȡ��Ũ�ȵ�SO2����NH4��2SO4��NH4HSO4���塣
Ϊ�˲ⶨ������NH4��2 SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00mL��������120�����ң�ʹ����ȫ���ݳ�[(NH4)2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200��]������й�ʵ���������£���״������
SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00mL��������120�����ң�ʹ����ȫ���ݳ�[(NH4)2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200��]������й�ʵ���������£���״������
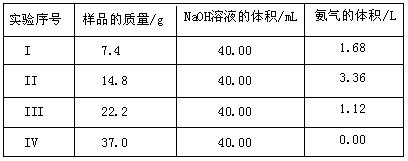
��1��ʵ��������йط�Ӧ�����ӷ���ʽΪ
��
��2����I������ֱ���Ʋ⣺��״����3.7g��Ʒ����ͬ��ʵ��ʱ�����ɰ��������Ϊ
L��
��3���Լ���û������(NH4)2SO4��NH4HSO4�����ʵ���֮�� ��
��4���������NaOH��Һ�����ʵ���Ũ��Ӧѡ��� �����ݣ��ɴ����NaOH��Һ�����ʵ���Ũ��Ϊ ��
Ϊ�˲ⶨ������NH4��2
 SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00mL��������120�����ң�ʹ����ȫ���ݳ�[(NH4)2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200��]������й�ʵ���������£���״������
SO4��NH4HSO4�����������ɣ��ֳ�ȡ����Ʒ�ķݣ��ֱ������ͬŨ�ȵ�NaOH��Һ��40.00mL��������120�����ң�ʹ����ȫ���ݳ�[(NH4)2SO4��NH4HSO4�ķֽ��¶Ⱦ�����200��]������й�ʵ���������£���״������
��1��ʵ��������йط�Ӧ�����ӷ���ʽΪ

��
��2����I������ֱ���Ʋ⣺��״����3.7g��Ʒ����ͬ��ʵ��ʱ�����ɰ��������Ϊ
L��
��3���Լ���û������(NH4)2SO4��NH4HSO4�����ʵ���֮�� ��
��4���������NaOH��Һ�����ʵ���Ũ��Ӧѡ��� �����ݣ��ɴ����NaOH��Һ�����ʵ���Ũ��Ϊ ��
��1��H+ + OH�� = H2O NH4+ + OH- =" " NH3 + H2O
��2�� 0.84 L����3�� 1:4 ����4�� �� 5.0 mol/L
��2�� 0.84 L����3�� 1:4 ����4�� �� 5.0 mol/L
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

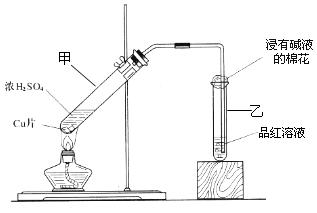
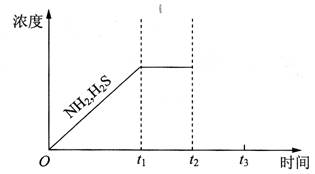
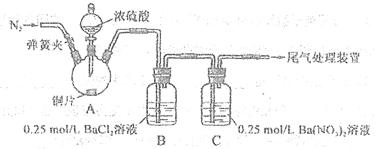
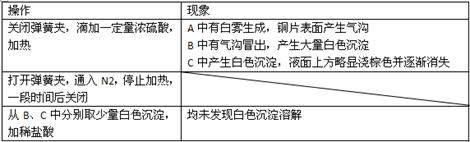


 �ʣ���ȷ�ķ�����
�ʣ���ȷ�ķ�����