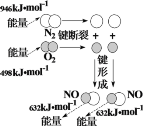��Ŀ����
����Ŀ���л���ѧ֪ʶ��������Ӧ�ù㷺��
��1�����ࡢ��֬�͵������Ƕ����Ժ�ֲ����ʳ���еĻ���Ӫ�����ʡ�
�������й�˵���У���ȷ����________________��
A���ޡ��顢ľ�ġ���˿����Ҫ�ɷֶ�����ά��
B����֬�Dz���������ߵ�Ӫ������
C���������������ڷ���ˮ���������ɰ�����
D����������������
E�����ۡ���ά�ء������ʶ�����Ȼ�߷��ӻ�����
F������炙�����Ǧ��Һ���뵽��������Һ�У������ʶ��ܴ���Һ������
��������������Ҫ����ĵ��ǣ�������һ��Ӫ�����ʣ����������ƾ��ȹ�ҵ�ϡ�д�������Ƿ���������Ӧ�Ļ�ѧ����ʽ��_____________________________________________��
��2��ƻ���᳣������ˮ���ǹ������Ӽ�����ṹ��ʽΪ![]() ���÷����й����ŵ�����Ϊ________________�����Ժʹ������ʷ���__________��Ӧ�������Է�����������ˮ���������ᣬ��������ʹ��ˮ��ɫ����������Ľṹ��ʽΪ_________________��
���÷����й����ŵ�����Ϊ________________�����Ժʹ������ʷ���__________��Ӧ�������Է�����������ˮ���������ᣬ��������ʹ��ˮ��ɫ����������Ľṹ��ʽΪ_________________��
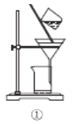
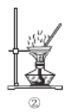
��3��ʵ���Һϳ����������IJ������£���Բ����ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4����118����77.1����
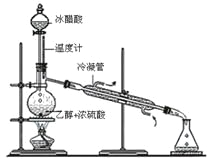
������ƿ�г��˼����Ҵ���Ũ����������⣬��Ӧ���뼸�����Ƭ����Ŀ����_____________��
���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ��ʹﵽ�˸÷�Ӧ���ȣ����ﵽ��ѧƽ��״̬������������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬���У�����ţ�___________________��
A����λʱ�������1mol����������ͬʱ����1molˮ
B����λʱ�������1mol����������ͬʱ����1mol����
C����λʱ�������1mol�Ҵ���ͬʱ����1mol����
D������Ӧ���������淴Ӧ���������
E��������и����ʵ�Ũ�Ȳ��ٱ仯
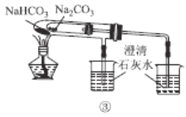
��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ��
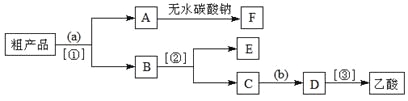
�Լ�a��______�����뷽������_______�����뷽������________���Լ�b��________��
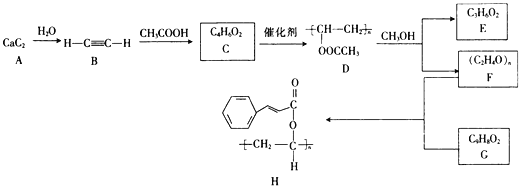
��д��C �� D ��Ӧ�Ļ�ѧ����ʽ________________________________________________��
���𰸡�BCEF CH2OH(CHOH)4CHO + 2Ag(NH3)2OH ![]() 2Ag��+ CH2OH(CHOH)4COONH4+3NH3+ H2O �Ȼ����ǻ� ��������ȡ���� HOOC��CH=CH��COOH ��ֹ��ƿ�е�Һ�屩�� BDE ����Na2CO3��Һ ��Һ ���� ��Ũ������ 2CH3COONa + H2SO4�� 2CH3COOH + Na2SO4
2Ag��+ CH2OH(CHOH)4COONH4+3NH3+ H2O �Ȼ����ǻ� ��������ȡ���� HOOC��CH=CH��COOH ��ֹ��ƿ�е�Һ�屩�� BDE ����Na2CO3��Һ ��Һ ���� ��Ũ������ 2CH3COONa + H2SO4�� 2CH3COOH + Na2SO4
��������
��1����A.�ޡ��顢ľ�ĵ���Ҫ�ɷ�����ά�أ���˿����Ҫ�ɷ��ǵ����ʣ�A����
B.���ࡢ��֬���������У���֬�Dz���������ߵ�Ӫ�����ʣ�B��ȷ��
C.��������������ˮ������ղ����ǰ����ᣬC��ȷ��
D.���������ζ������ۡ���ά�ص�û����ζ��D����
E.���ۡ���ά�ء������ʶ�����Ȼ�߷��ӻ����E��ȷ��
F.����識��뵽��������Һ�У������ʷ�������������Һ������������Ǧ���뵽��������Һ�У������ʷ������Զ�������F��ȷ��
��ѡBCEF��
�������ǵĽṹ��ʽΪCH2OH��CHOH��4CHO����ṹ�к�ȩ��������������Һ����������Ӧ����Ӧ�Ļ�ѧ����ʽΪCH2OH(CHOH)4CHO + 2Ag(NH3)2OH![]() 2Ag��+ CH2OH(CHOH)4COONH4+3NH3+ H2O��
2Ag��+ CH2OH(CHOH)4COONH4+3NH3+ H2O��
��2������ƻ����Ľṹ��ʽ��֪��ƻ�����к��еĹ����ŵ�����Ϊ�ǻ����Ȼ�����Ϊ���Ȼ������Կ��봼�����ʷ���������Ӧ��������ӦҲ��һ��ȡ����Ӧ���ṹ�к��ǻ������ǻ�����̼ԭ�ӵ���̼������Hԭ�ӣ���ƻ���ᷢ����ȥ��Ӧ���������ᣬ��������ʹ��ˮ��ɫ��������Ľṹ��ʽΪHOOC��CH=CH��COOH��
��3���ٶ�Һ���������ʱ��Ϊ��ֹҺ�����ʱ�ľ�����������Ҫ���뼸�����Ƭ�����������Ƭ��Ŀ���ǣ���ֹ��ƿ�е�Һ�屩�С�
��A. ��λʱ�������1mol����������ͬʱ����1molˮ��ֻ��ʾ����Ӧ������˵����Ӧ�ﵽƽ��״̬��
B. ��λʱ�������1mol����������ͬʱ����1mol���ᣬ��ʾ�����淴Ӧ������ȣ���˵����Ӧ�ﵽƽ��״̬��
C. ��λʱ�������1mol�Ҵ���ͬʱ����1mol���ᣬֻ��ʾ����Ӧ������˵����Ӧ�ﵽƽ��״̬��
D. ����Ӧ���������淴Ӧ����������ǿ��淴Ӧ�ﵽƽ��״̬�ı��ʱ�־��
E. ������и����ʵ�Ũ�Ȳ��ٱ仯�ǿ��淴Ӧ�ﵽƽ��״̬��������־��
��ѡBDE��
���������������к����Ҵ��������ˮ�����ȼ�����Լ�aΪ����Na2CO3����Ϊ����Na2CO3��Һ�ܽ��������������ܽ�ȣ������Ҵ�����������ת��Ϊ�����ƣ����뱥��Na2CO3��Һ�������ó��ֲַ����ʷ��뷽����Ϊ��Һ��B��ˮ���к��Ҵ��������ƣ�Ϊ�˽�B�гɷַ�������Ҵ������뷽����Ϊ����EΪ�Ҵ���CΪ��������Һ�����������������ᣬ���ݡ�ǿ�������ᡱ�ĸ��ֽⷴӦ���ɣ���������ӷ�����C�м������ᣬ�����������Ʒ�Ӧ�������ᣬ���D����õ����ᡣ
��C��D�Ļ�ѧ����ʽΪ2CH3COONa+H2SO4��2CH3COOH+Na2SO4��

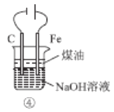
����Ŀ������ʾ��ͼ�뻯ѧ����������ݲ�������ǣ�ˮ����������Ӧ���ӷ��ű�ʾ��
A | B | C | D |
|
|
|
|
NaCl����ˮ | ����������ʴԭ�� | ͭпԭ��ع����ԭ�� | N2��O2��Ӧ�����仯 |
NaCl��Na+ + Cl�� | ������Fe �� 2e����Fe2+ | �ܷ�Ӧ��Zn + Cu2+��Zn2+ + Cu | N2(g) + O2(g)��2NO(g)��H����180 kJ��mol��1 |
A.AB.BC.CD.D
����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ���ش��������⣺
��1��ȷ����5.0 g���������������ʵ���Ʒ�����ʲ������ᷴӦ�������250 mL������Һ���õ��IJ����������ձ�������������ͷ�ιܡ�___________________��
��2���ζ�ʱ����0.2000 mol��L��1���������ζ�������Һ����ȡ����Һ10.00 mLӦѡ��_________����(����ĸ)��
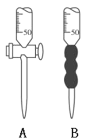
��3���ζ������У��۾�Ӧע��___________________________________���Լ�����ָʾ������ȷ�жϵζ��յ��������________________________________��
��4�����±���֪����2�����������������ƫ������ܵ�ԭ����_________��
�ζ����� | ������Һ���(mL) | ������� | |
�ζ�ǰ�Ŀ̶�(mL) | �ζ���Ŀ̶�(mL) | ||
��һ�� | 10.00 | 0.40 | 20.50 |
�ڶ��� | 10.00 | 2.10 | 24.20 |
������ | 10.00 | 4.10 | 24.00 |
a����ƿ�ô���Һ��ϴ
b���ζ���������ƿ����Һ����ƿ��
c���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������ʧ
d���ζ�����ʱ�����Ӷ���
��5�����ݱ������ݣ����㱻���ռ���Һ�����ʵ���Ũ����_________���ռ���Ʒ�Ĵ�����_________��