��Ŀ����
���ǵ����Ϻ����ḻ��ԭ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á�
��1��25��ʱ��0.1mol/LNH4NO3��Һ��ˮ�ĵ���̶� ������ڡ��������ڡ���С�ڡ��� 0.1mol/L NaOH��Һ��ˮ�ĵ���̶ȡ�
��2������0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������ϣ������Һ��2c(NH4+)��c(NO3��)��������Һ������Ũ���ɴ�С��˳���� ��
��3��������ʱ��(N2H4)Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ�����ⶨ16g������������Ӧ�зų�284kJ����������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4����ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��
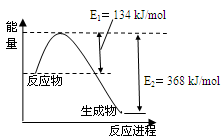
��֪��N2(g)��O2(g)��2NO(g) ��H����180kJ/mol
2NO (g)��O2(g)��2NO2(g) ��H����112.3kJ/mol
��Ӧ��2NO(g)��2CO(g) N2(g)��2CO2(g)�ġ�H�� ��
N2(g)��2CO2(g)�ġ�H�� ��
��1��25��ʱ��0.1mol/LNH4NO3��Һ��ˮ�ĵ���̶� ������ڡ��������ڡ���С�ڡ��� 0.1mol/L NaOH��Һ��ˮ�ĵ���̶ȡ�
��2������0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������ϣ������Һ��2c(NH4+)��c(NO3��)��������Һ������Ũ���ɴ�С��˳���� ��
��3��������ʱ��(N2H4)Ϊȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ�����ⶨ16g������������Ӧ�зų�284kJ����������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4����ͼ��1mol NO2��1mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��

��֪��N2(g)��O2(g)��2NO(g) ��H����180kJ/mol
2NO (g)��O2(g)��2NO2(g) ��H����112.3kJ/mol
��Ӧ��2NO(g)��2CO(g)
 N2(g)��2CO2(g)�ġ�H�� ��
N2(g)��2CO2(g)�ġ�H�� ����1������ ��2��c(NO3��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)
��3��2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol��4����760.3kJ/mol
��3��2N2H4(g)��2NO2(g)��3N2(g)��2H2O (g) ��H����1136kJ/mol��4����760.3kJ/mol
��������� ��1���ᡢ������ˮ�ĵ��롢��ˮ����δٽ�ˮ�ĵ��롣
��2��0.1mol/L NaOH��Һ��0.2mol/LNH4NO3��Һ�������Ϻ�c(Na+)=0.05mol/L��c(NO3-)=0.1mol/L����2c(NH4+)��c(NO3��)��c(NH4+)>0.05mol/L������غ�ʽΪc(Na+)+c(NH4+)+c(H+)=c(NO3-)+c(OH-)����c(Na+)��c(NO3-)��c(NH4+)��c(OH��)��c(H+)������c(NO3��)��c(NH4+)��c(Na+)��c(OH��)��c(H+)��
��3��2N2H4��2NO2��3N2��2H2O��16gN2H4Ϊ0.5mol������2molN2H4��Ӧ����1136kJ��
��4����ͼ��ɵ�NO2(g)+CO(g)
 CO2(g)+NO(g) ��H����234kJ/mol���ɸ�˹���ɵ�2NO(g)��2CO(g)
CO2(g)+NO(g) ��H����234kJ/mol���ɸ�˹���ɵ�2NO(g)��2CO(g) N2(g)��2CO2(g)�ġ�H=����234��2+180+112.3��kJ/mol=��760.3kJ/mol��
N2(g)��2CO2(g)�ġ�H=����234��2+180+112.3��kJ/mol=��760.3kJ/mol��
��ϰ��ϵ�д�
�����Ŀ
 2NH3��g����H=-38��6kJ?mol-1
2NH3��g����H=-38��6kJ?mol-1