��Ŀ����
���û�ѧ��Ӧԭ���о�����Ԫ�صĵ��ʼ��仯��������Ҫ���塣
��1���ϳɰ���ӦN2 (g)+3H2(g) 2NH3(g)����H��0�����ں��¡���ѹ��������ƽ����ϵ��ͨ���������ƽ�� �ƶ�����������ҡ���������ʹ�ô�����������Ӧ�ġ�H________������� ����С�� ���䡱����
2NH3(g)����H��0�����ں��¡���ѹ��������ƽ����ϵ��ͨ���������ƽ�� �ƶ�����������ҡ���������ʹ�ô�����������Ӧ�ġ�H________������� ����С�� ���䡱����
��2����Na2C2O4��Һ��������ʯ���Һ�� ɫ���������ӷ���ʽ��ʾ���ָ������ԭ�� ��
��3��pH��ͬ�İ�ˮ���������ƣ��ֱ�������ˮϡ����ԭ����Һ��100������ϡ�ͺ�������Һ��pH�ֱ�Ϊm��n����m n��ѡ�>����<����=������
��4����������Ѱ����ʵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl�DNH4ClΪ�������Һ��������ȼ�ϵ�ء���д���õ�ص�������Ӧʽ ��
��5������ȼ����ֵ�ߡ�ʵ���ã��ڳ��³�ѹ�£�4gH2��ȫȼ������Һ̬ˮ���ų�571��6kJ���������ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��6��ij�¶ȣ�t�棩ʱ�����0.01mol?L��1��NaOH��Һ��pH��11���ڴ��¶��£���pH��a��H2SO4��ҺVaL��pH��b��NaOH��ҺVbL��ϣ������û��ҺΪ���ԣ���a��b��12����Va��Vb�� ��
��1���ϳɰ���ӦN2 (g)+3H2(g)
 2NH3(g)����H��0�����ں��¡���ѹ��������ƽ����ϵ��ͨ���������ƽ�� �ƶ�����������ҡ���������ʹ�ô�����������Ӧ�ġ�H________������� ����С�� ���䡱����
2NH3(g)����H��0�����ں��¡���ѹ��������ƽ����ϵ��ͨ���������ƽ�� �ƶ�����������ҡ���������ʹ�ô�����������Ӧ�ġ�H________������� ����С�� ���䡱������2����Na2C2O4��Һ��������ʯ���Һ�� ɫ���������ӷ���ʽ��ʾ���ָ������ԭ�� ��
��3��pH��ͬ�İ�ˮ���������ƣ��ֱ�������ˮϡ����ԭ����Һ��100������ϡ�ͺ�������Һ��pH�ֱ�Ϊm��n����m n��ѡ�>����<����=������
��4����������Ѱ����ʵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl�DNH4ClΪ�������Һ��������ȼ�ϵ�ء���д���õ�ص�������Ӧʽ ��
��5������ȼ����ֵ�ߡ�ʵ���ã��ڳ��³�ѹ�£�4gH2��ȫȼ������Һ̬ˮ���ų�571��6kJ���������ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ ��
��6��ij�¶ȣ�t�棩ʱ�����0.01mol?L��1��NaOH��Һ��pH��11���ڴ��¶��£���pH��a��H2SO4��ҺVaL��pH��b��NaOH��ҺVbL��ϣ������û��ҺΪ���ԣ���a��b��12����Va��Vb�� ��
��15�֣���1��������
��2������C2O42-+H2O
 HC2O4-+OH-
HC2O4-+OH-��3��>
��4��N2+8H++6e��=2NH4+
(5)H2��g��+1/2 O2(g)=H2O��l�� ����H=-285.8kJ?mol��1
��6��1:10
��

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ
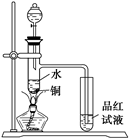
 2��ϡ���Ṳ������ȡ����
2��ϡ���Ṳ������ȡ����