��Ŀ����
����Ŀ��ij���dz��Ը���Ϊԭ�����ǣ�ͬʱ�õ������ĸ��������Ը����������ۺ����á�����������߾���Ч�棬���һ��ܷ�ֹ������Ⱦ�������������£�

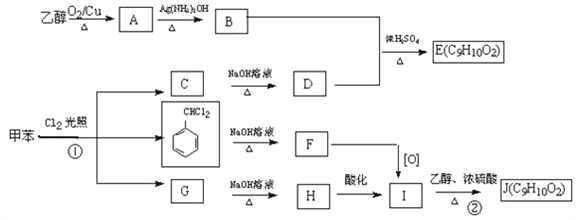
��֪ʯ���ѽ��ѳ�Ϊ����C����Ҫ������E����Һ�ܷ���������Ӧ��G�Ǿ��й���ζ��Һ�壬����գ�
(1) B�����ƣ�_________________��D�й����ŵ����ƣ�___________________��
(2) д��C�����Ӿ۷�Ӧ�ķ���ʽ��___________________��
(3) D��E�Ļ�ѧ����ʽ��_____________________��F��G�Ļ�ѧ����ʽ��_____________________��
(4)��֪HCOOCH3Ҳ���й���ζ�����������G�Ĺ�ϵ����Ϊ_________________����F�Ĺ�ϵ����Ϊ��_________________��
��ͼΪʵ������ȡG��װ��ͼ��ͼ��a�Լ�����Ϊ________________��ʵ����������a��G�ķ���Ϊ_________________��

���𰸡� ������ �ǻ� ![]() 2CH3CH2OH��O2
2CH3CH2OH��O2![]()
![]() 2CH3CHO��2H2O CH3COOH��CH3CH2OH
2CH3CHO��2H2O CH3COOH��CH3CH2OH![]() CH3COOCH2CH3 ��H2O ͬϵ�� ͬ���칹�� ����Na2CO3��Һ ��Һ
CH3COOCH2CH3 ��H2O ͬϵ�� ͬ���칹�� ����Na2CO3��Һ ��Һ
������������������֮��õ���ά�أ���ά��ˮ������ղ���Ϊ�����ǣ�����A����ά�أ�B�������ǣ��������ھƻ�ø�������·�Ӧ�����Ҵ�����D���Ҵ���D��������������EΪCH3CHO��E��һ������������Ӧ����FΪCH3COOH��G�Ǿ�����ζ��Һ�壬F��D����������Ӧ����GΪCH3COOCH2CH3��C��ˮ�ڴ��������·�Ӧ�����Ҵ���CΪ��ϩ��
(1) B�������ǣ�D���Ҵ���������Ϊ�ǻ����ʴ�Ϊ�������ǣ��ǻ���
(2)��ϩ�����Ӿ۷�Ӧ�ķ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(3)�Ҵ�����������ȩ �Ļ�ѧ����ʽΪ2CH3CH2OH��O2![]() 2CH3CHO��2H2O���������Ҵ������Ļ�ѧ����ʽΪCH3COOH��CH3CH2OH
2CH3CHO��2H2O���������Ҵ������Ļ�ѧ����ʽΪCH3COOH��CH3CH2OH![]() CH3COOCH2CH3 ��H2O���ʴ�Ϊ��2CH3CH2OH��O2
CH3COOCH2CH3 ��H2O���ʴ�Ϊ��2CH3CH2OH��O2![]() 2CH3CHO��2H2O��CH3COOH��CH3CH2OH
2CH3CHO��2H2O��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3 ��H2O��
CH3COOCH2CH3 ��H2O��
(4)HCOOCH3��CH3COOCH2CH3�ṹ���ƣ�����Ϊͬϵ�HCOOCH3��CH3COOH�ķ���ʽ��ͬ������Ϊͬ���칹�壬ʵ������ȡ��������ʵ���У��ñ���Na2CO3��Һ�ռ����ɵ�����������ʵ��������÷�Һ�ķ�����������������̼������Һ���ʴ�Ϊ��ͬϵ�ͬ���칹�壻����Na2CO3��Һ�� ��Һ��