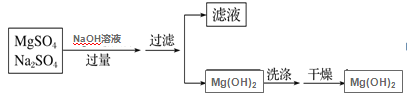
��Ŀ����
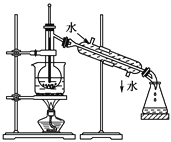
����Ŀ����Ϣʱ�������Ĵ������������Ի��������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���ɫ����M�ͽ���E���������¿�ͼ��ʾת������ø�Ч��ˮ��K2EO4��
������ʱ����ѧʽ����ѧ����ʽ�е�M��E��������Ӧ��Ԫ�ط��ű�ʾ����
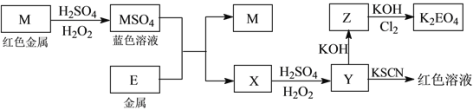
��1��д��M����ϡH2SO4��H2O2���Һ�����ӷ���ʽ��_______________________________��
��2������X�������ӵķ�����������__________________________________________________��
��3��ijͬѧȡX����Һ�ڿ����з��ú��ữ������KI�͵�����Һ����Һ��Ϊ��ɫ��д���������仯������ص����ӷ���ʽ��_______________________________��____________________________________��
��4����MSO4����ɫ��Һ��һϵ�в������Ի����ɫ���壬��Щ�������õ���ʵ���������˾ƾ��ơ�����������̨�⣬����Ҫ�õ��IJ���������________��________�� __________����д�������ƣ���
��5��ijͬѧ����H2��ԭMO���ⶨM�����ԭ����������ͼ�Dzⶨװ��ʾ��ͼ��A���Լ������ᡣ
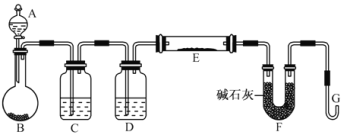
������B��Ӧװ��___________��װ��D������_________________________��
�����Ӻ�װ�ò�����װ�õ������Ժ�Ӧ���������ȷ�Ӧ��E����������Aƿ����μ���Һ���� ��
___________________________��������֮�仹Ӧ���еIJ�����_________________________��
���𰸡�Cu+H2O2+2H+= Cu2++2 H2OȡX��Һ�������Թ��У����뼸��KSCN��Һ����Һ���Ժ�ɫ�������Թ��м��뼸��������ˮ����Һ�Ժ�ɫ��4Fe2+ + O2 +4H+��4Fe3+ +2H2O2Fe3+ + 2I-�� 2Fe2+ + I2�ձ�©��������п����Zn������ˮ����������H2��Aƿ����μ���Һ�����H2�Ĵ���
��������
��1��M�Ǻ�ɫ��������M��ͭ��˫��ˮ����ǿ�����ԣ���ϡ������Һ���ܰ�ͭ������������ͭ����˫��ˮ�Ļ�ԭ������ˮ����Ӧ�����ӷ���ʽ����Cu+H2O2+2H+= Cu2++2H2O����ȷ����Cu+H2O2+2H+=Cu2++2H2O��
��2��Y�ܺ�KSCN��Һ��Ӧ����Һ�Ժ�ɫ������Y�к��������ӣ����E�ǽ���������������ͭ��Ӧ����ͭ��������������X�������������������Ӿ��л�ԭ�ԣ��ݴ˿��Լ��飬������X�������ӵķ�������������ȡX��Һ�������Թ��У����뼸��KSCN��Һ����Һ���Ժ�ɫ�������Թ��м��뼸��������ˮ����Һ�Ժ�ɫ����ȷ����ȡX��Һ�������Թ��У����뼸��KSCN��Һ����Һ���Ժ�ɫ�������Թ��м��뼸��������ˮ����Һ�Ժ�ɫ��
��3���������Ӿ��л�ԭ�ԣ��ڿ������ױ��������������������������Ӿ��������ԣ��ܰѵ⻯���������ɵ��ʵ⣬������������ɫ����Ӧ�����ӷ���ʽ����4Fe2++O2 +4H+��4Fe3++2H2O�� 2Fe3+ +2I-��2Fe2+ +I2 ����ȷ���� 4Fe2++O2 +4H+��4Fe3++2H2O�� 2Fe3++2I-��2Fe2+ +I2 ��
��4��������ͭ��Һ�еõ�����ͭ����IJ�������������Щ�������õ���ʵ���������˾ƾ��ơ�����������̨�⣬����Ҫ�õ��IJ������������ձ���©��������������ȷ�����ձ���©������������
��5����Bװ�����Ʊ������ģ���Һ©����װ����������ƿB��ʢ�ŵ���п�������������ӷ����������ɵ������к����Ȼ����ˮ���������Cװ����ʢ�б���ʳ��ˮ�������Ȼ��⣬Dװ����ʢ��Ũ���ᣬ����ˮ������������������ȷ����п����Zn���� ����ˮ����������H2��
������װ���л����п�����ʵ��ǰ��Ҫ���������ž��������������ȴ�Aƿ����μ���Һ�塣����Ϊ�����ǿ�ȼ�����壬��ȼǰ��Ҫ���������Ĵ��ȣ�������������֮�仹Ӧ���еIJ����Ǽ��������Ĵ�������ȷ������Aƿ����μ���Һ���� ����H2�Ĵ�����

����Ŀ���ζ�ʵ���ǻ�ѧѧ��������Ҫ�Ķ���ʵ��֮һ�������ĵζ�ʵ��������к͵ζ���������ԭ��Ӧ�ζ��������ζ��ȵȡ�
��1������к͵ζ�������0.1000mol/LHCl����Һ�ⶨijNaOH��Һ�����ʵ���Ũ�ȣ��÷�̪��ָʾ��������������ƽ��ʵ�飬���ݼ�¼���£�
ʵ����� | ����NaOH��Һ�����/mL | 0.1000mol/LHCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 25.01 |
2 | 25.00 | 1.56 | 26.56 |
3 | 25.00 | 0.21 | 25.20 |
���������NaOH��Һ�����ʵ���Ũ��Ϊ_____________________________��С���������λ����
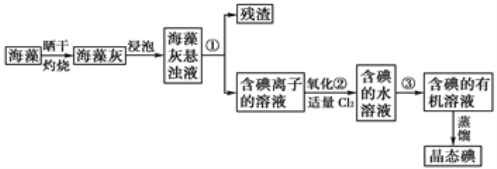
��2��������ԭ�ζ��������Ѿ��п��������������IJⶨ�����ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�����(������SO2����)�ķ������£�

ע��ʵ���м��������Ŀ���ǽ�Na2S2O5ȫ��ת����SO2���ζ������з����ķ�Ӧ�ǣ�I2 + SO2 + 2H2O ![]() 2HI + H2SO4
2HI + H2SO4
���ζ�ʱ��I2��ҺӦװ��__________(��������������)ʽ�ζ����У��жϵζ��յ�ķ����ǣ����������һ�ε���Һʱ����Һ��_________________________���ұ���30s������
��ʵ�����ı�I2��Һ25.00mL��������Ʒ�п��������IJ�����(������SO2����)Ϊ___________g/L��
���������λ���ɲⶨ���ƫ�ߵ���_____________��
A���ζ�����ʱ���Գ�����Һ�в���HI���������� B���ζ�ǰƽ�ӣ��ζ�����
C��ʢװ��I2��Һ�ĵζ���������ˮϴ����δ��ϴ
D���ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ
��3�������ζ������ζ����ͱ��ζ����������ȵζ�����ָʾ��������������ܡ��ο��±��е����ݣ�����AgNO3�ζ�NaSCN��Һ����ѡ�õ�ָʾ����_____________(��ѡ����ĸ)��
������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
��ɫ | �� | dz�� | �� | ש�� | �� |
Ksp | 1.77��10-10 | 5.35��10-13 | 1.21��10-16 | 1.12��10-12 | 1.0��10-12 |
A��NaCl B��NaBr C��NaCN D��Na2CrO4
����Ŀ��ijͬѧ����֪���ʵ���Ũ�ȵ�NaOH�ⶨδ֪���ʵ���Ũ�ȵ����ᣬ��20.00 ![]() �������������ƿ��,���μ�2-3�η�̪��ָʾ��,��NaOH����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
�������������ƿ��,���μ�2-3�η�̪��ָʾ��,��NaOH����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
ʵ���� |
| �ζ����ʱ, | ������������/ |
1 | 0.10 | 22.62 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.80 | 20.00 |
�� �ζ��ﵽ�յ�ı�־��____________________________��
�� ������������,�ɼ�����������Ũ��ԼΪ_______(������λ��Ч����)��
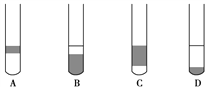
�� �ų���ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е�________,Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

�� ������ʵ����,���в���(����������ȷ)����ɲⶨ���ƫ�ߵ���________(����ĸ���)��
A. �ζ��յ����ʱ����
B. ��ʽ�ζ���ʹ��ǰ,ˮϴ��δ�ô���������ϴ
C. ��ƿˮϴ��δ����
D. ������![]() �������
�������![]() ����
����
E. ��ʽ�ζ��ܼ��첿��������,�ζ�����ʧ