��Ŀ����
��10�֣���֪X��Y��Z��W�Ƕ����������ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ���γɵ�����ֻ��һ�����ӣ�Z��W��Ԫ�����ڱ��д������ڵ�λ�ã����ǵĵ����ڳ����¾�Ϊ��ɫ���壻Yԭ�ӵ��������������ڲ��������2����
��1��д��������ĸ������Ԫ�ص����ƣ�X ��Y ��Z ��W ��
��2��X���ʺ�Z������һ�������·�Ӧ���ɻ�����E��д���÷�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ������ ��Y���ʺ�W���ʣ��������ڼ��������·�Ӧ���ɻ�����F����F���ӵĿռ乹��Ϊ ������ʽΪ ��
��3��������Ԫ�ؿ����X��Y��Z��Wԭ�Ӹ�����Ϊ5��1��1��3�Ļ�����û������ˮ��Һ������ŨNaOH��Һ��Ӧ�����ӷ���ʽΪ��
___________________________________________________________________
��1��д��������ĸ������Ԫ�ص����ƣ�X ��Y ��Z ��W ��
��2��X���ʺ�Z������һ�������·�Ӧ���ɻ�����E��д���÷�Ӧ�Ļ�ѧ����ʽ����ע����Ӧ������ ��Y���ʺ�W���ʣ��������ڼ��������·�Ӧ���ɻ�����F����F���ӵĿռ乹��Ϊ ������ʽΪ ��
��3��������Ԫ�ؿ����X��Y��Z��Wԭ�Ӹ�����Ϊ5��1��1��3�Ļ�����û������ˮ��Һ������ŨNaOH��Һ��Ӧ�����ӷ���ʽΪ��
___________________________________________________________________
��1���� ̼ �� ����ÿ��1�֣�
 ��2��N2+3H2 2NH3 ��2�֣�ûд������������Ÿ���1�֣���ֱ���ͣ�
��2��N2+3H2 2NH3 ��2�֣�ûд������������Ÿ���1�֣���ֱ���ͣ�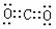 ������1�֣�
������1�֣�
��3��NH4+ + HCO3- + 2OH- = NH3 ��+ 2H2O + CO32- ��2��,д��NH3�qH2O�����֣���
 ��2��N2+3H2 2NH3 ��2�֣�ûд������������Ÿ���1�֣���ֱ���ͣ�
��2��N2+3H2 2NH3 ��2�֣�ûд������������Ÿ���1�֣���ֱ���ͣ�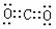 ������1�֣�
������1�֣���3��NH4+ + HCO3- + 2OH- = NH3 ��+ 2H2O + CO32- ��2��,д��NH3�qH2O�����֣���
��

��ϰ��ϵ�д�
 �ŵ������ϵ�д�
�ŵ������ϵ�д� 53������ϵ�д�
53������ϵ�д�
�����Ŀ

