��Ŀ����
����Ŀ�������л�����ת����ϵ��ͼ��ʾ(���±仯�У�ijЩ��Ӧ����������δ����)��A����Ȼ�л��߷��ӻ����D��һ����Ҫ�Ļ���ԭ�ϡ�����ͬ�����£�G�����ܶ���������44����
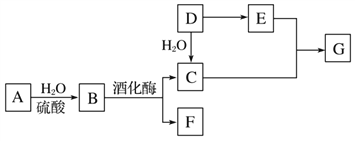
(1)D�����ƣ�_____________��
(2)C��E��Ӧ�Ļ�ѧ����ʽ��_____________________________________��
(3)����Aת����������B���ɣ����к�ˮ��Һ������Ҫ������Լ���_____________��
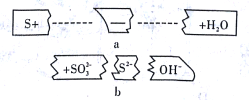
(4)ij��X����Է���������D��F֮�ͣ�������̼���������֮����5��1������˵����ȷ����________(����ĸ)��
A��X ������ˮ���뱽��Ϊͬϵ�� B��X�����ȶ��������²���ֽ�
C��X��ͬ���칹����ܴ���4���� D��X���ܺ���ˮ�����ӳɷ�Ӧ
���𰸡� ��ϩ CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O ����������ͭ(��������Һ) C
CH3COOCH2CH3��H2O ����������ͭ(��������Һ) C
��������A����Ȼ�л��߷��ӻ����������������ˮ���B��B�ھƻ�ø������������C��F������AΪ��C6H10O5��n��BΪC6H12O6��G�����ܶ���������44������G����Է�������Ϊ88��D��һ����Ҫ�Ļ���ԭ�ϣ�D��ˮ�ӳɵ�C������CΪCH3CH2OH��FΪCO2����DΪCH2=CH2��D������EΪCH3COOH��C��E����������Ӧ����GΪCH3COOCH2CH3����X����Է���������D��F֮�ͣ���Ϊ72��������̼���������֮����5��1����XΪC5H12��
��1����������ķ�����֪��DΪCH2=CH2��D�й����ŵ�����Ϊ̼̼˫����
��2��C��E����������Ӧ����CH3COOCH2CH3����Ӧ�Ļ�ѧ����ʽCH3CH2OH+CH3COOH![]() CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
��3������Aת����������B���ɣ������������ǵĴ��ڣ����к�ˮ��Һ������Ҫ������Լ�������������ͭ����������Һ����
��4��XΪC5H12���DZ���������A��C5H12������ˮ���뱽������ͬϵ��Ĺ�ϵ����A����B��C5H12�ڸ����»�ֽ⣬��B����C��C5H12��һ��ͬ���칹��Ϊ2��2-�������飬���ĸ�������C��ȷ��D����������ˮ�������ӳɷ�Ӧ����D����ѡC��

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�