��Ŀ����
3��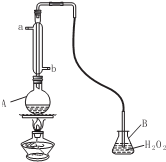 ��ҵ������β���е�SO2����һ�ֿ�����Ⱦ�ͬʱҲ��һ����Ҫ�Ļ���ԭ�ϣ����ð����շ�����ѭ�����ã�����Ա��Ϊ����
��ҵ������β���е�SO2����һ�ֿ�����Ⱦ�ͬʱҲ��һ����Ҫ�Ļ���ԭ�ϣ����ð����շ�����ѭ�����ã�����Ա��Ϊ������1���ð�ˮ����β���е�SO2������������泥�������Ӧ�ķ���ʽΪ2NH3•H2O+SO2=��NH4��2SO3+H2O
��2������SO2�����գ�����Һ�е�NH4 HSO3Ũ�ȴﵽһ���̶Ⱥ��������м���ϡ���ᣬ�ų�SO2ѭ��ʹ�ã�ͬʱ������һ�ָ����NH4��2SO4���ѧʽ�����������ʣ�
��3���ڳ��е���ˮ�Ĵ����У�SO2�����ڴ����ŷ�ǰ���Ȼ���ˮ�����Ȼ���ˮ�е�������SO2��Ӧ�Ļ�ѧ����ʽΪCl2+SO2+2H2O=H2SO4+2HCl
��4����������ͨ����Ϊ���������ӵ����Ѿ��У���ɱ���������������塢�ܽ⡢���Ʒ�ζ����������ã������˹����Ķ�������������к����ҹ����ұ��涨���Ѿ���SO2�����ʹ����Ϊ 0.25g•L-l����ͼΪij��ȤС������Ѿ��е�SO2�����ռ��������װ�ã�
����������ˮ�Ľ���Ϊ��b���a����b����
��A�м���300.00ml���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����B��H2O2��ȫ��Ӧ���仯ѧ����ʽΪSO2+H2O2=H2SO4
�۳�ȥB�й�����H2O2��Ȼ����0.0900mol/L NaOH����Һ���еζ����ζ����յ�ʱ������NaOH��Һ25.00ml�������Ѿ���SO2����Ϊ��0.24g/L��
���� ��1�����������������ˮ��Ӧ����������泥����ԭ���غ���ƽ��д��ѧ����ʽ��
��2��β���е�SO2�ð����գ����ð�ˮ����β���е�SO2����Ӧ����������炙���������泥�������炙���������������ᷴӦ��������李����������ˮ��
��3��������ˮ��SO2��Ӧ������������
��4����Ϊ�˳����ȴ���壬Ӧ���¿ڽ�ˮ��
�ڶ���������л�ԭ�ԣ��ܹ���������ⷴӦ�������ᣬ�ݴ�д����Ӧ�Ļ�ѧ����ʽ��
�۸��ݹ�ϵʽ2NaOH��H2SO4��SO2���������Ƶ����ʵ������������������������ټ���������Ѿ��еĶ�����������
��� �⣺��1�����������������ˮ������������泥����ԭ���غ���ƽ��д��ѧ����ʽΪ��2NH3•H2O+SO2=��NH4��2SO3+H2O��
�ʴ�Ϊ��2NH3•H2O+SO2=��NH4��2SO3+H2O��
��2��β���е�SO2�ð����գ����ð�ˮ����β���е�SO2����ѧ����ʽΪ��SO2+2NH3+H2O=��NH4��2SO3����NH4��2SO3+SO2+H2O=2NH4HSO3��������Һ��NH4HSO3�ﵽһ��Ũ�Ⱥ��������ᷴӦ���ų���SO2��ѭ�����ã����ɵģ�NH4��2SO4�������ʣ���ѧ����ʽ�ǣ�2NH4HSO3+H2SO4=��NH4��2SO4+2SO2��+2H2O����NH4��2SO3+H2SO4=��NH4��2SO4+SO2��+H2O��
�ʴ�Ϊ����NH4��2SO4��
��3��SO2�����ڴ����ŷ�ǰ���Ȼ���ˮ�����Ȼ���ˮ�е�������SO2��Ӧ�Ļ�ѧ����ʽΪ��Cl2+SO2+2H2O=H2SO4+2HCl��
�ʴ�Ϊ��Cl2+SO2+2H2O=H2SO4+2HCl��
��4���ٸ�������A�Ĺ����֪������AΪ�����ܣ���������ͨˮ�����������ͨˮ��������Ч����ѣ�����Ӧ�ý�ˮ��Ϊb��
�ʴ�Ϊ��b��
��˫��ˮ���������ԣ��ܹ��������������������ᣬ��Ӧ�Ļ�ѧ����ʽΪ��SO2+H2O2=H2SO4��
�ʴ�Ϊ��SO2+H2O2=H2SO4��
�۸���2NaOH��H2SO4��SO2��֪SO2������Ϊ��$\frac{1}{2}$����0.0900mol/L��0.025L����64g/mol=0.072g�������Ѿ��еĶ���������Ϊ��$\frac{0.72g}{0.3L}$=0.24g/L��
�ʴ�Ϊ��0.24��
���� ���⿼����̽�����ʵ���ɡ��������ʵĺ����ķ�������Ŀ�Ѷ��еȣ������漰�˶����������ʡ��к͵ζ��ļ��㣬Ҫ��ѧ������̽��������ɡ��������ʺ����ķ�������ȷ��������Ļ�ѧ���ʼ��к͵ζ��IJ������������㷽��������������ѧ�����Ӧ����ѧ֪ʶ������

| A�� | ����ˮ������KMnO4�����������ϩ | |
| B�� | ����ˮ������KMnO4���𱽺ͱ���ͬϵ�� | |
| C�� | ����ˮ��FeCl3��Һ������KMnO4�����Լ��𱽷���Һ�;ƾ���Һ | |
| D�� | ��±�������ȼ���NaOH��Һ�����ȣ��ټ�AgNO3���ɳ��������ɸ�����ɫȷ��±��ԭ�ӵ����� |
| A�� | CH3Cl | B�� | CH3CHBrCH3 | C�� |  | D�� |  |
| A�� | ±�� | B�� | ��������Ԫ�� | C�� | �ڶ�����Ԫ�� | D�� | �ڢ�A��Ԫ�� |
| A�� | �����������䣬�ʵ����������Ũ�Ƚ��ӿ컯ѧ��Ӧ���� | |
| B�� | ���������²��䣬�ʵ�����CaCO3���������ӿ컯ѧ��Ӧ���� | |
| C�� | ��Ӧ�����У���ѧ��Ӧ���ʽ���������С | |
| D�� | һ�������·�Ӧ���ʸı䣬��H��0���� |
| A�� | H2S03 | B�� | Ca��OH��2 | C�� | KCl03 | D�� | CO2 |
| A�� | CH3-CH2Cl | B�� | CH3CH=CHBr | C�� | CH3C CCH3 | D�� | CH3CH=C��CH3��2 |
 ��1���۲�ͼA��B��C���ش��������⣺
��1���۲�ͼA��B��C���ش��������⣺