��Ŀ����
Ϊ��СCO2�Ի�����Ӱ��,�ڳ�������̼����ͬʱ,�����ǿ��CO2�������õ��о���T1��ʱ,��9 mol CO2��12 mol H2����3 L�ܱ�������,������ӦCO2(g)+3H2(g) CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��
CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��
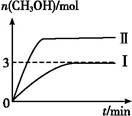
 CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��
CH3OH(g)+H2O(g) ��H��0,������CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ,ƽ��ʱ������ѹǿΪp1���ı�ijһ�������½���������Ӧ,CH3OH�����ʵ�����ʱ��仯�����ߢ���ʾ������˵��������ǣ� ��
| A�����ߢ��Ӧ�������ı�������ѹǿ |
| B��T2��ʱ,������Ӧƽ�ⳣ��Ϊ0.42,��T2��T1 |
| C����T1��,����ʼʱ�������г���5 mol CO2��5 mol H2��5 mol CH3OH(g)��5 mol H2O(g),���ƽ��ǰv(��)��v(��) |
D����T1��,����ʼʱ����������4.5 mol CO2��6 mol H2,ƽ��ʱ������ѹǿp=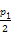 |
D
������ѹǿ,���ʼӿ�,ƽ������,��CH3OH�����ʵ�������,A��;��ͼ֪,T1���´�ƽ��ʱCH3OHΪ3 mol,��������CO2Ϊ6 mol��H2Ϊ3 mol��H2OΪ3 mol,�����ʵ�ƽ��Ũ��:CO2Ϊ2 mol/L��H2Ϊ1 mol/L��H2OΪ1 mol/L��CH3OHΪ1 mol/L,���ƽ�ⳣ��Ϊ0.5,��÷�Ӧ�������,�����������ƶ�,ƽ�ⳣ����С,����,��T2��ʱƽ�ⳣ��Ϊ0.42,��T2��T1,B��;��T1��,����ʼʱ�������г���5 mol CO2��5 mol H2��5 mol CH3OH(g)��5 mol H2O(g),Q=0.36��0.5,���������,��ƽ��ǰv(��)��v(��),C��;��T1��,����Ͷ�Ϸ�ʽ������Ӧ���ת�������,��p=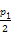 ,�������ڸ÷�Ӧ�������������С�ķ�Ӧ,����ʼ���뷴Ӧ������ʵ����ٵ�,��ת����С,��p��
,�������ڸ÷�Ӧ�������������С�ķ�Ӧ,����ʼ���뷴Ӧ������ʵ����ٵ�,��ת����С,��p��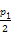 ,D����
,D����
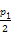 ,�������ڸ÷�Ӧ�������������С�ķ�Ӧ,����ʼ���뷴Ӧ������ʵ����ٵ�,��ת����С,��p��
,�������ڸ÷�Ӧ�������������С�ķ�Ӧ,����ʼ���뷴Ӧ������ʵ����ٵ�,��ת����С,��p��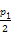 ,D����
,D����
��ϰ��ϵ�д�
�����Ŀ
 2HI(g)����H<0�����ﵽƽ���t0ʱ�����ֻ�����������ʵ���������ı�ijһ��Ӧ������ʹ������ѹǿ����(��ͼ��ʾ)������˵����ȷ���� (����)
2HI(g)����H<0�����ﵽƽ���t0ʱ�����ֻ�����������ʵ���������ı�ijһ��Ӧ������ʹ������ѹǿ����(��ͼ��ʾ)������˵����ȷ���� (����)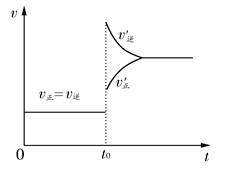
 CH3OH��g����һ�ܱ������дﵽƽ�⡣�����������������������������ά��H2��Ũ�Ⱥ��������¶Ȳ��䣬��ԭƽ����Ƚϴﵽ��ƽ��ʱCO��ת���ʽ���������
CH3OH��g����һ�ܱ������дﵽƽ�⡣�����������������������������ά��H2��Ũ�Ⱥ��������¶Ȳ��䣬��ԭƽ����Ƚϴﵽ��ƽ��ʱCO��ת���ʽ��������� CH3OH(g)��
CH3OH(g)��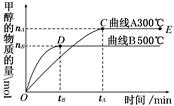
 pC(g)+qD(g)ƽ�ⳣ��ΪK������˵����ȷ����( )
pC(g)+qD(g)ƽ�ⳣ��ΪK������˵����ȷ����( ) pC��g����qD�У�A��C������ɫ���壬�ﵽƽ�������������ȷ���ǣ���������
pC��g����qD�У�A��C������ɫ���壬�ﵽƽ�������������ȷ���ǣ���������