��Ŀ����
��298 K��1.01��105 Pa��,��22 g CO2ͨ��750 mL 1 mol��L-1NaOH��Һ�г�ַ�Ӧ,��÷�Ӧ�ų�x kJ����������֪�ڸ�������,1 mol CO2ͨ��1 L 2 mol��L-1 NaOH��Һ�г�ַ�Ӧ�ų�y kJ����������CO2��NaOH��Һ��Ӧ����NaHCO3���Ȼ�ѧ����ʽΪ(����)
A��CO2(g)+NaOH(aq) NaHCO3(aq) ��H=-(2y-x)kJ��mol-1 NaHCO3(aq) ��H=-(2y-x)kJ��mol-1 |
B��CO2(g)+NaOH(aq) NaHCO3(aq) ��H=-(2x-y)kJ��mol-1 NaHCO3(aq) ��H=-(2x-y)kJ��mol-1 |
C��CO2(g)+NaOH(aq) NaHCO3(aq) ��H=-(4x-y)kJ��mol-1 NaHCO3(aq) ��H=-(4x-y)kJ��mol-1 |
D��CO2(g)+NaOH(aq) NaHCO3(aq) ��H=-(8x-2y)kJ��mol-1 NaHCO3(aq) ��H=-(8x-2y)kJ��mol-1 |
C
����

���з�Ӧ�У�Q2>Q1���� (����)
| A��H2(g)��F2(g)=2HF(g)����H����Q1 kJ��mol��1 H2(g)��Cl2(g)=2HCl(g)����H����Q2 kJ��mol��1 |
| B��2H2(g)��O2(g)=2H2O(l)����H����Q1 kJ��mol��1 2H2(g)��O2(g)=2H2O(g)����H����Q2 kJ��mol��1 |
| C��NaOH(aq)��HCl(aq)=NaCl(aq)��H2O(l)��H����Q1 kJ��mol��1 NaOH(aq)��CH3COOH(aq)=CH3COONa(aq)��H2O(l)��H����Q2 kJ��mol��1 |
| D��S(s)��O2(g)=SO2(g)����H����Q1 kJ��mol��1 |
��ʢ��NH4HCO3��ĩ��С�ձ�����ʢ����������Ĵ��ձ��С�Ȼ����С�ձ��м������ᣬ��Ӧ���ң����������̡��ɴ˿ɼ� �� ��
| A��NH4HCO3������ķ�Ӧ�Ƿ��ȷ�Ӧ |
| B���÷�Ӧ�У�����ת��Ϊ�����ڲ������� |
| C����Ӧ�������������������������� |
| D����Ӧ���Ȼ�ѧ����ʽΪNH4HCO3��HCl�D��NH4Cl��CO2����H2O����H����Q kJ��mol��1 |
ij��ѧ�����ö�������(CeO2)��̫���������½�H2O��CO2ת��ΪH2��CO����������£�
mCeO2 (m��x)CeO2��xCe��xO2
(m��x)CeO2��xCe��xO2
(m��x)CeO2��xCe��xH2O��xCO2 mCeO2��xH2��xCO
mCeO2��xH2��xCO
����˵������ȷ���ǣ� ��
A���ù�����CeO2û������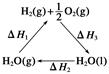 |
| B���ù���ʵ����̫������ѧ�ܵ�ת�� |
| C��ͼ�Ц�H1����H2����H3 |
| D����CO��O2���ɵļ���ȼ�ϵ�صĸ�����ӦʽΪCO��4OH����2e��=CO32-��2H2O |
����(P)��Cl2������Ӧ����PCl3��PCl5����Ӧ���̺������Ĺ�ϵ����ͼ��ʾ��ͼ�еĦ�H��ʾ����1 mol��������ݡ���֪PCl5�ֽ�����PCl3��Cl2���÷ֽⷴӦ�ǿ��淴Ӧ������˵����ȷ���ǣ� ��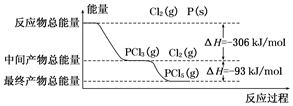
| A�������������䣬�����¶�������PCl5������ |
| B����Ӧ2P(s)��5Cl2(g)=2PCl5(g)��Ӧ�ķ�Ӧ�ȡ���H����798 kJ/mol |
| C��P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽΪ��2P(s)��3Cl2(g)=2PCl3(g)����H����306 kJ/mol |
| D�������������䣬����2PCl5(g)=2P(s)��5Cl2(g)����H��Ӧ������ѹǿ��PCl5��ת���ʼ�С����H��С |
ͬ��ͬѹ��,���и����Ȼ�ѧ����ʽ��,��H1<��H2���ǣ� ��
| A��S(g)+O2(g)=SO2(g) ��H1S(s)+O2(g)=SO2(g) ��H2 |
B�� H2(g)+ H2(g)+ Cl2(g)="HCl(g)" ��H1H2(g)+Cl2(g)="2HCl(g)" ��H2 Cl2(g)="HCl(g)" ��H1H2(g)+Cl2(g)="2HCl(g)" ��H2 |
| C��2H2(g)+O2(g)=2H2O(g) ��H12H2(g)+O2(g)=2H2O(l) ��H2 |
D��C(s)+ O2(g)="CO(g)" ��H1C(s)+O2(g)=CO2(g) ��H2 O2(g)="CO(g)" ��H1C(s)+O2(g)=CO2(g) ��H2 |
��֪����CH3OH��g����H2O��g��=CO2��g����3H2��g������H����49.0 kJ��mol��1
��CH3OH��g����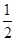 O2��g��=CO2��g����2H2��g����H����192.9 kJ��mol��1
O2��g��=CO2��g����2H2��g����H����192.9 kJ��mol��1
����˵����ȷ���� ����������
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ������������������������� |
C�����ݢ���֪��Ӧ��CH3OH��l����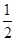 O2��g��=CO2��g����2H2��g���Ħ�H>��192.9 kJ��mol��1 O2��g��=CO2��g����2H2��g���Ħ�H>��192.9 kJ��mol��1 |
| D����Ӧ���е������仯����ͼ��ʾ |

��֪����1 mol H2�����л�ѧ������ʱ��Ҫ����436 kJ����������1 mol Cl2�����л�ѧ������ʱ��Ҫ����243 kJ������������Hԭ�Ӻ�Clԭ���γ�1 mol HCl����ʱ�ͷ�431 kJ������������������ȷ���� ����������
| A��������������Ӧ�����Ȼ���������Ȼ�ѧ����ʽ��H2��g����Cl2��g��=2HCl��g�� |
| B��������������Ӧ����2 mol�Ȼ������壬��Ӧ�Ħ�H����183 kJ��mol��1 |
| C��������������Ӧ����2 mol�Ȼ������壬��Ӧ�Ħ�H����183 kJ��mol��1 |
| D��������������Ӧ����1 mol�Ȼ������壬��Ӧ�Ħ�H����183 kJ��mol��1 |
��֪�������£�0.01 mol/L MOH��Һ��pHΪ10��MOH(aq)��H2SO4(aq)��Ӧ����1 mol���εĦ�H����24.2 kJ��mol��1��ǿ����ǿ���ϡ��Һ���к���Ϊ��H����57.3 kJ��mol��1����MOH��ˮ��Һ�е���Ħ�HΪ(����)
| A����69.4 kJ��mol��1�������� | B����45.2 kJ��mol��1 |
| C����69.4 kJ��mol��1 | D����45.2 kJ��mol��1 |