��Ŀ����
���ڹ�����ռ����Ҫ��λ��
��1��NH3�ĵ���ʽΪ
��2��ʵ��������ȡ�����Ļ�ѧ����ʽΪ �����ɵ����������պ��Ũ����IJ����������飬����������ˮ����Һ�����ԣ������ӷ���ʽ��ʾԭ�� ��
��Һ������Ũ���ɴ�С��˳��Ϊ
��3����������ȡ�������ɽ�Ũ��ˮ����������������У���װ����ͼ�����ϻ�ѧ����ӻ�ѧƽ��ĽǶȽ��ͣ�

��4����SO2����ͨ���Ȼ�����Һ��δ���г������ɣ�����ͨ��NH3������ְ�ɫ��������д����Ӧ�����ӷ���ʽ ������SO2����ͨ���ữ�����ᱵ��Һ��Ҳ������ɫ�����������ӷ���ʽ����˵��
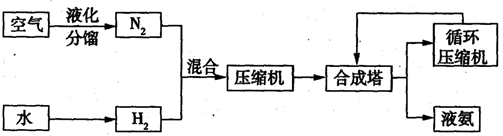
��5�������£�N2H4���ǵ������ֳ���������ڿ�ѧ����������������Ҫ��Ӧ�á�
�¡�������ȼ�ϵ����һ�ּ��Ե�أ��õ�طŵ�ʱ�������ķ�Ӧʽ ��
��6������������ŷŻ�Կ��������Ⱦ��������������ȥ�����еĵ�������������������в���������Ȼ�������ͨ��ʯ�����У�ʹ֮ת��Ϊ����ơ���֪ij����������NO��NO2��ɣ���n(NO)��n(NO2)=1��3.д���������շ���ȥ��������Ļ�ѧ����ʽ___________________
��1�� (1��)
(1��)
��2��2NH4Cl +Ca(OH)2 CaCl2+2NH3 ��+2H2O
(2��)NH������H��O
CaCl2+2NH3 ��+2H2O
(2��)NH������H��O NH����H��O��H��(1��)
NH����H��O��H��(1��)
c��Cl���� ��c��NH4+�� ��c��H������ c��OH���� ��1�֣�
��3�� NH3��H��O NH����H��O
NH����H��O NH������OH-
NH������OH-
NaOH����ˮ�������룬ʹc��OH��������ƽ��������NH3�ķ����ƶ���NH3 �ݳ���2�֣�
��4�� 2NH3+SO2+H2O+Ba2+=BaSO3��+2NH4+ (1��) (�𰸺���������)
3SO2+2H2O+2NO3-+3Ba2+=3BaSO4��+2NO ��+4H+(1��) (�𰸺���������)
��5��N2H4�� 4e��+ 4OH�� ��N2+4H2O (2��)
��6��3O2 + 2NO + 6NO2 + 4Ca(OH)2=4Ca(NO3)2 + 4H2O (2��)
��������
�����������1��Nԭ�ӷֱ���3��Hԭ���γ�һ�Թ��õ��ӣ�����ʽΪ��
��2��ʵ������Ca(OH)2��NH4Cl��ȡNH3����ѧ����ʽΪ��2NH4Cl
+Ca(OH)2 CaCl2+2NH3
��+2H2O�����ɵIJ���ΪNH4Cl��ˮ�������ԣ����ӷ���ʽΪ��NH4++H2O
CaCl2+2NH3
��+2H2O�����ɵIJ���ΪNH4Cl��ˮ�������ԣ����ӷ���ʽΪ��NH4++H2O  NH3•H2O+H+��NH4+ˮ��Ũ��С��Cl‾����Һ��������H+Ũ�ȴ���OH‾Ũ�ȣ�����c��Cl���� ��c��NH4+��
��c��H������ c��OH������
NH3•H2O+H+��NH4+ˮ��Ũ��С��Cl‾����Һ��������H+Ũ�ȴ���OH‾Ũ�ȣ�����c��Cl���� ��c��NH4+��
��c��H������ c��OH������
��3����ˮ�д���ƽ�⣺NH3��H��O NH����H��O
NH����H��O  NH������OH-��NaOH����ˮ�������룬ʹc��OH�����������ܽ�ʱ���ȣ�ƽ��������NH3�ķ����ƶ���NH3
�ݳ���
NH������OH-��NaOH����ˮ�������룬ʹc��OH�����������ܽ�ʱ���ȣ�ƽ��������NH3�ķ����ƶ���NH3
�ݳ���
��4��SO2��H2O��NH3��Ӧ������SO32‾��SO32‾��Ba2+�������BaSO3 ���������ӷ���ʽΪ��2NH3+SO2+H2O+Ba2+=BaSO3��+2NH4+ ��SO2��NO3‾��H+����ΪSO42‾��SO42‾��Ba2+�������BaSO4���������ӷ���ʽΪ��3SO2+2H2O+2NO3-+3Ba2+=3BaSO4��+2NO ��+4H+��
��5��N2H4�ڼ���������ʧ��������N2��H2O����ƽ�ɵõ缫����ʽ��N2H4�� 4e��+ 4OH�� ��N2+4H2O��
��6��n(NO)��n(NO2)=1��3����ʽ��NO��NO2��ϵ��Ϊ1:3���ɵû�ѧ����ʽ��3O2 + 2NO + 6NO2 + 4Ca(OH)2=4Ca(NO3)2 + 4H2O��
���㣺���⿼�����ʽ�������ʵ�����Ʒ�������Ũ�ȱȽϡ���ѧ����ʽ�����ӷ���ʽ�Լ��缫����ʽ����д��������ʵĵ��롢�����ˮ�⡣


 2NH3�ġ�H
2NH3�ġ�H