��Ŀ����
���ڹ�����ռ����Ҫ�ĵ�λ�����������̽����
��1��������������ˮ����ͨ�����ȵ�̿������ˮú����C��s��+H2O��g��?H2��g��+CO��g����H=+131.3kJ/mol����S=+133.7J/K
�÷�Ӧ�ڵ������ܷ��Է� ����ܻ��
��2����֪��400��ʱ��N2 ��g��+3H2��g��?2NH3��g����H��0 ��K1=0.5��
��2NH3��g��?N2 ��g��+3H2��g����K2= ������ֵ����
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV��N2���� V��N2���������������=������ȷ������
��3����֪��Ӧ��CO��g��+2H2��g��?CH3OH ��g����H��0
a���÷�Ӧ��һ�������´ﵽƽ����ڱ�֤H2Ũ�Ȳ��������£�����������������Ը���ƽ�ⳣ�����ж�ƽ��
A��������Ӧ�����ƶ� B�����淴Ӧ�����ƶ� C�����ƶ�
b�����÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
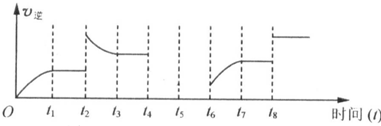
t2ʱ��ƽ�ⳣ��K��t1ʱ����ȿ��� ������ĸ���ţ�
A������ B����С C�����䣮
��1��������������ˮ����ͨ�����ȵ�̿������ˮú����C��s��+H2O��g��?H2��g��+CO��g����H=+131.3kJ/mol����S=+133.7J/K
�÷�Ӧ�ڵ������ܷ��Է�
��2����֪��400��ʱ��N2 ��g��+3H2��g��?2NH3��g����H��0 ��K1=0.5��
��2NH3��g��?N2 ��g��+3H2��g����K2=
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV��N2����
��3����֪��Ӧ��CO��g��+2H2��g��?CH3OH ��g����H��0
a���÷�Ӧ��һ�������´ﵽƽ����ڱ�֤H2Ũ�Ȳ��������£�����������������Ը���ƽ�ⳣ�����ж�ƽ��
A��������Ӧ�����ƶ� B�����淴Ӧ�����ƶ� C�����ƶ�
b�����÷�Ӧ���淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��

t2ʱ��ƽ�ⳣ��K��t1ʱ����ȿ���
A������ B����С C�����䣮
��������1�����ݷ�Ӧ�ܷ��Է����е��оݣ���H-T��S��0����Ӧ�Է����У��������������㣻
��2���ٻ�Ϊ�淴Ӧ�Ļ�ѧ��Ӧƽ�ⳣ����Ϊ������
�ڸ���Ũ���غ�ƽ�ⳣ��֮��Ĺ�ϵ���жϷ�Ӧ��״̬��
��3��a������Ӱ�컯ѧƽ���ƶ����������ش�
b���淴Ӧ���������ԭ��������¶ȡ�Ũ�ȵı仯��Ҳ������ѹǿ�ı仯���������Ǵ����ı仯��
��2���ٻ�Ϊ�淴Ӧ�Ļ�ѧ��Ӧƽ�ⳣ����Ϊ������
�ڸ���Ũ���غ�ƽ�ⳣ��֮��Ĺ�ϵ���жϷ�Ӧ��״̬��
��3��a������Ӱ�컯ѧƽ���ƶ����������ش�
b���淴Ӧ���������ԭ��������¶ȡ�Ũ�ȵı仯��Ҳ������ѹǿ�ı仯���������Ǵ����ı仯��
����⣺��1����H-T��S=131.3KJ/mol-T��133.7��0.001KJ/��K?mol�����ڵ����£���ֵһ���Ǵ���0�ģ����Բ����ڵ������Է����У��ʴ�Ϊ����
��2���ٷ�Ӧ2NH3��g��?N2 ��g��+3H2��g���ͷ�ӦN2 ��g��+3H2��g��?2NH3��g���ǻ�Ϊ���淴Ӧ��ƽ�ⳣ����Ϊ�������ʴ˷�Ӧ��ƽ�ⳣ����2���ʴ�Ϊ��2��
��һ��ʱ���N2��H2��NH3�����ʵ����ֱ�Ϊ4mol/L��2mol/L��4mol/Lʱ��Qc=
=0.5=K1�����Ը�״̬��ƽ��״̬�����淴Ӧ������ȣ��ʴ�Ϊ��=��
��3����֤H2Ũ�Ȳ��������£����������������������������Ũ�Ⱦ����䣬��Qc��Ȼ�ǵ���K����Ȼ��ƽ��״̬����ѡC��
��4���淴Ӧ���������ԭ������������¶ȣ��������ʵ�Ũ�Ȼ�������ѹǿ���������Ǵ��������Ƕ��ڷ�ӦCO��g��+2H2��g��?CH3OH ��g����H��0�������¶�ʱ K��С����B��ȷ�����������ʵ�Ũ�ȡ�����ѹǿ���Ǽ������ʱ��K�����䣬��ѡ��BC��
��2���ٷ�Ӧ2NH3��g��?N2 ��g��+3H2��g���ͷ�ӦN2 ��g��+3H2��g��?2NH3��g���ǻ�Ϊ���淴Ӧ��ƽ�ⳣ����Ϊ�������ʴ˷�Ӧ��ƽ�ⳣ����2���ʴ�Ϊ��2��
��һ��ʱ���N2��H2��NH3�����ʵ����ֱ�Ϊ4mol/L��2mol/L��4mol/Lʱ��Qc=
| 42 |
| 4��23 |
��3����֤H2Ũ�Ȳ��������£����������������������������Ũ�Ⱦ����䣬��Qc��Ȼ�ǵ���K����Ȼ��ƽ��״̬����ѡC��
��4���淴Ӧ���������ԭ������������¶ȣ��������ʵ�Ũ�Ȼ�������ѹǿ���������Ǵ��������Ƕ��ڷ�ӦCO��g��+2H2��g��?CH3OH ��g����H��0�������¶�ʱ K��С����B��ȷ�����������ʵ�Ũ�ȡ�����ѹǿ���Ǽ������ʱ��K�����䣬��ѡ��BC��
���������⿼��ѧ���йػ�ѧƽ����й�֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ
 2NH3�ġ�H
2NH3�ġ�H