��Ŀ����
��(Ti)����Ϊ��������֮��ĵ����������Ѱ�(TiO2)��Ŀǰ��õİ�ɫ���ϡ��Ʊ�TiO2��Ti��ԭ�����������ҹ�������������������λ������Fe2O3��������(��Ҫ�ɷ�ΪFeTiO3)��ȡTiO2���������£�
ͼ1-5
(1)Ti��ԭ������Ϊ22��Tiλ��Ԫ�����ڱ��е�_________���ڣ���________�塣
(2)����ټ�Fe��Ŀ���ǣ�__________________________________________________��
�������ȴ��Ŀ���ǣ�_____________________________________________________��
(3)�����Ʊ�TiO2�Ĺ����У��������õĸ�������______________________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����_________������
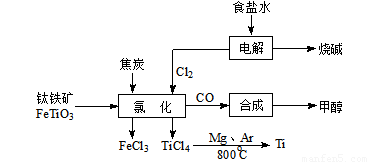
(4)�ɽ��ʯ(TiO2)��ȡ����Ti���漰�IJ���Ϊ��
![]()
��֪����C(S)+O2(g)![]() CO2(g);��H=-393.5 kJ��mol-1
CO2(g);��H=-393.5 kJ��mol-1
��2CO(g)+O2(g)![]() 2CO2(g);��H=-566 kJ��mol-1
2CO2(g);��H=-566 kJ��mol-1
��TiO2(S)+2Cl2(g)![]() TiCl4(s)+O2(g);��H=+141 kJ��mol-1
TiCl4(s)+O2(g);��H=+141 kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s)![]() TiCl4(s)+2CO(g)�Ħ�H=______________��
TiCl4(s)+2CO(g)�Ħ�H=______________��
��ӦTiCl4+2Mg![]() 2MgCl2+Ti��Ar�����н��е�������_______________________��
2MgCl2+Ti��Ar������������_______________________��
˼·����:������һ�����͵������ͼ�Ʊ��⡣��������Ի���֪ʶ�����գ�����Ҫ����ѧ��֪ʶ���ۺ�Ӧ��������
(1)��ԭ�������ƶ�Ԫ�������ڱ��е�λ�ã��ؼ�����ϤԪ�����ڱ��Ľṹ������������ֹԪ�ص�ԭ����������������λ�õȡ�Ti��ԭ������Ϊ22������Ϥ��Ca(ԭ������Ϊ20)�������Ca��Ԫ�����ڱ��е�λ�ã������ƶ�Tiλ�ڵ������ڣ��ڢ�B�塣
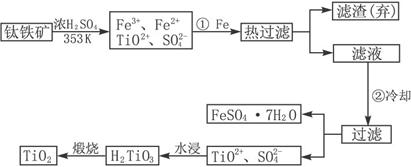
(2)�ɿ�ͼ��ʾ���̷�����֪��Ҫ��TiO2+��Fe2+��Fe3+���룬��Ҫ�ȼ������۽�Fe3+��ԭΪFe2+�����ȹ��˳�ȥ�������ٽ���Һ��ȴʹFeSO4��7H2O������
(3)�Ʊ�TiO2�����еĸ�����FeSO4��7H2O�ڹ�ũҵ�������ճ��������н϶��Ӧ�á��ڷ����H2TiO3���ķ�Һ�У�������������TiO2+��Fe2+���ɼ���ʯ��(��̼��ơ��ϼ�)����H+��Ũ�ȣ�ʹ֮ת��Ϊ��������������ѭ�����á�
(4)��Ӧ��ֻ�뷴Ӧ��ʼ̬����̬�йأ��������ķ�Ӧ���е�;���ء�����֪�Ȼ�ѧ����ʽ��Ӽ���+2����-�ڵ�TiO2(s)+2Cl2(g)+2C(s)![]() TiCl4(s)+2CO(g)���䦤H=141 kJ�� mol-1+2��(-393.5 kJ�� mol-1)-(-566 kJ�� mol-1)=-80 kJ�� mol-1����Ϊ�ڸ����£�Mg(Ti)������е�O2(��CO2��N2)���ã�������Mg��ԭTiCl4ʱҪ�ڶ�������(Ar)�н��С�
TiCl4(s)+2CO(g)���䦤H=141 kJ�� mol-1+2��(-393.5 kJ�� mol-1)-(-566 kJ�� mol-1)=-80 kJ�� mol-1����Ϊ�ڸ����£�Mg(Ti)������е�O2(��CO2��N2)���ã�������Mg��ԭTiCl4ʱҪ�ڶ�������(Ar)�н��С�
�𰸣�(1)4 ��B (2)��Fe2+��ԭΪFe3+
����(����롢��õ�)FeSO4��7H2O
(3)FeSO4��7H2O ʯ��(��̼��ơ��ϼ�)
(4)-80 kJ�� mol-1
��ֹ������Mg(Ti)������е�O2(��CO2��N2)����

(13��) ��(Ti)����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�
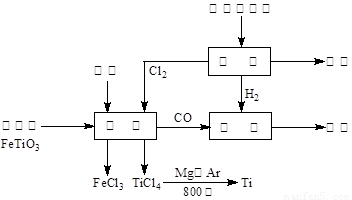
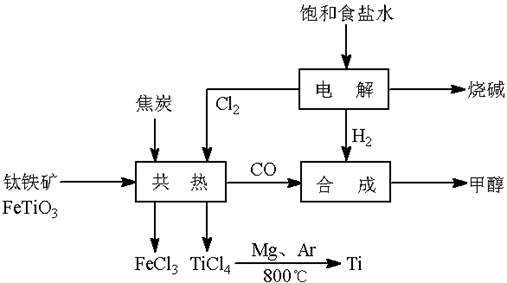
��1����ⱥ��ʳ��ˮʱ�������ĵ缫��ӦΪ ��
��2��д���������뽹̿��Cl2�����Ƶ����Ȼ��ѵĻ�ѧ����ʽ________________________��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s)����H = �C 641 kJ/mol
��Ti(s) + 2Cl2(g)��TiCl4(s)����H = �C770 kJ/mol
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s)����H�� ��
��Ӧ2Mg(s) + TiCl4(s) 2MgCl2(s) + Ti(s)����Ar�����н��е�������
��
2MgCl2(s) + Ti(s)����Ar������������
��
��4����������ҵ���У��ϳ�96 t �״�����������H2 t (�������������������ʵ��κ���ʧ)��
��5���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH + 3O2 + 4OH����2CO32�� + 6H2O����ȼ�ϵ�ط�����Ӧʱ��������Һ��pH (���������С�����䡱)���õ���и����ϵĵ缫��Ӧ��________________________________________________��

 2MgCl4��Ti��Ar������������____________________��
2MgCl4��Ti��Ar������������____________________�� CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol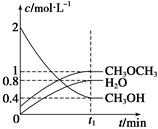

 2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
��
2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
�� ��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��
��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��