��Ŀ����
��(Ti)����Ϊ��������֮��ĵ����������Ѱ�(TiO2)��Ŀǰ��õİ�ɫ���ϡ��Ʊ�TiO2��Ti��ԭ�����������ҹ�������������������λ������Fe2O3��������(��Ҫ�ɷ�ΪFeTiO3)��ȡTiO2���������£�
(1)Ti��ԭ������Ϊ22,Tiλ��Ԫ�����ڱ��е�_________���ڣ���_________�塣
(2)����ټ�Fe��Ŀ����___________________________��
�������ȴ��Ŀ��________________________________��
(3)�����Ʊ�TiO2�Ĺ����У��������õĸ�������_________�����dzɱ��ͷ����ۺ��������أ���Һ��Ӧ����_________������
(4)�ɽ��ʯ(TiO2)��ȡ����Ti���漰���IJ���Ϊ��
TiO2![]() TiCl4
TiCl4![]() Ti
Ti
��֪����C(s)+O2(g)====CO2(g)����H=-393.5 kJ��mol-1
��2CO(g)+O2(g)====2CO2(g)����H=-566 kJ��mol-1
��TiO2(s)+2Cl2(g)====TiCl4(s)+O2(g)����H=+141 kJ��mol-1
��TiO2(s)+2Cl2(g)+2C(s)====TiCl4(s)+2CO(g)�Ħ�H=__________��
��ӦTiCl4+2Mg====2MgCl2+Ti��Ar�����н��е�������____________________��
����������Ҫ������ͨ�����ͼ���Ĺ۲����⣬�������������ȡ������Ϣ����ѡ��͵����Լ������֪ʶ�������Ǽ���Ǩ��ת������õ�����Ĵ𰸡��ص㿼��ѧ���Ĺ۲�������˼ά������(3)���з�ҺӦ��ͼʾˮ����������H2TiO3ͬʱ���ɵ�H2SO4��Һ(TiOSO4+2H2O====H2TiO3+H2SO4)��Ӧ����������ʴ�����(4)С�⿼�鷴Ӧ�ȵļ��㡣����˹���ɣ�������֪��,�ڿ������2C(s)+O2(g)====2CO(g)�Ħ�H=2��(-393.5)+566=-221 kJ��mol-1,�ɴ˽�Ϣۿ���������ʦ�H=-221+141=-80 kJ��mol-1��
�𰸣�(1)4 ��B
(2)��Fe3+��ԭ��Fe2+ ����(����롢��õ�)FeSO4��7H2O
(3)FeSO4��7H2O ʯ��(��̼��ơ��ϼ�)
(4)-80 kJ��mol-1 ��ֹ������Mg(Ti)������е�O2(��CO2��N2)����

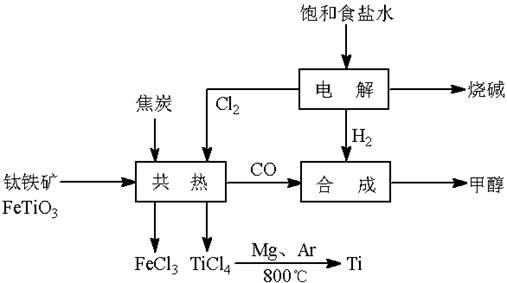
(13��) ��(Ti)����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�
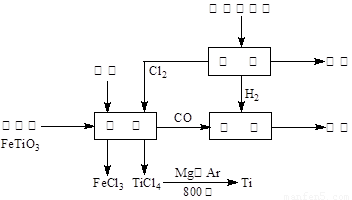
��1����ⱥ��ʳ��ˮʱ�������ĵ缫��ӦΪ ��
��2��д���������뽹̿��Cl2�����Ƶ����Ȼ��ѵĻ�ѧ����ʽ________________________��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s)����H = �C 641 kJ/mol
��Ti(s) + 2Cl2(g)��TiCl4(s)����H = �C770 kJ/mol
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s)����H�� ��
��Ӧ2Mg(s) + TiCl4(s) 2MgCl2(s) + Ti(s)����Ar�����н��е�������
��
2MgCl2(s) + Ti(s)����Ar������������
��
��4����������ҵ���У��ϳ�96 t �״�����������H2 t (�������������������ʵ��κ���ʧ)��
��5���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء���֪��ȼ�ϵ�ص��ܷ�ӦʽΪ��2CH3OH + 3O2 + 4OH����2CO32�� + 6H2O����ȼ�ϵ�ط�����Ӧʱ��������Һ��pH (���������С�����䡱)���õ���и����ϵĵ缫��Ӧ��________________________________________________��

 2MgCl4��Ti��Ar������������____________________��
2MgCl4��Ti��Ar������������____________________�� CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol
CH3OCH3(g)+H2O(g)����H=" -23.5" kJ/mol


 2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
��
2MgCl2(s)
+ Ti����Ar�����н��е������ǣ�
______________________________________
�� ��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��
��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ��
��