��Ŀ����
Ǧ�����ǵ��͵Ŀɳ��͵�أ���������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��
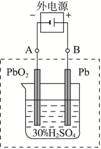
Ǧ����ʾ��ͼ
Pb+PbO2+4H++2![]()
![]() 2PbSO4+2H2O
2PbSO4+2H2O
�������������(�������⡢����������ԭ):
(1)�ŵ�ʱ�������ĵ缫��Ӧʽ��
____________________________________________________;���Һ��H2SO4��Ũ�Ƚ���_________________;�����·ͨ��1 mol����ʱ�������ϸ��������������
_________________g��
(2)����ȫ�ŵ�ľ�PbO2��Pbʱ��������ͼ���ӣ����һ��ʱ�������A�缫������
_____________________��B�缫������_____________________,��ʱǦ���ص��������ļ��Խ�__________________________________��
(1)PbO2+2e-+4H++![]() ====PbSO4+2H2O С 48
====PbSO4+2H2O С 48
(2)Pb PbO2 �Ի�
����:
�ڷ�Ӧ�����У���������H+���ʷ�ӦҺ��c(H+)�����͡���ȫ�ŵ���ٰ�ͼ���ӣ����γɵ��أ�A��Ϊ������B��Ϊ������������������PbO2����ȥ�����Դ��Ǧ���ص�����������ԭ���෴��

��ϰ��ϵ�д�
�����Ŀ
 Ǧ�����ǵ��͵Ŀɳ��͵�أ���������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��Pb+PbO2+4H++2SO2-4
Ǧ�����ǵ��͵Ŀɳ��͵�أ���������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��Pb+PbO2+4H++2SO2-4 Ǧ�����ǵ��͵Ŀɳ��͵�أ������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��Pb+PbO2+4H++2SO42-?2PbSO4+2H2O
Ǧ�����ǵ��͵Ŀɳ��͵�أ������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��Pb+PbO2+4H++2SO42-?2PbSO4+2H2O Ǧ�����ǵ��͵Ŀɳ��͵�أ����������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ��
Ǧ�����ǵ��͵Ŀɳ��͵�أ����������������Ƕ��Բ��ϣ�����ܷ�ӦʽΪ�� w.w.w.zxxk.c.o.m
w.w.w.zxxk.c.o.m 2PbSO4+2H2O
2PbSO4+2H2O