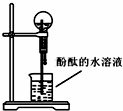
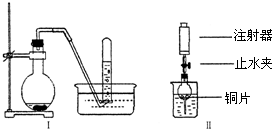
��Ŀ����
��200mLϡ������6.4g��ͭ�����ȵ������³�ַ�Ӧ����ԭ����ΪNO�����ⶨNO�����Ϊ1.12L����״������
��1��д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ��______
��2�������������Һ��HNO3�����ʵ���Ũ�ȼ��μӷ�Ӧ��ͭ��������
��3������12.5mol?L-1��Ũ����������500mL��ϡ���ᣮ�ɹ�ѡ��������У�
�ٲ���������ƿ���ձ��ܽ�ͷ�ιܢ���Ͳ��������ƽ��ҩ�ף����������У�����ʱ����Ҫʹ�õ�������______������ţ�����ȱ�ٵ�һ��������______��
��1��д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ��______
��2�������������Һ��HNO3�����ʵ���Ũ�ȼ��μӷ�Ӧ��ͭ��������
��3������12.5mol?L-1��Ũ����������500mL��ϡ���ᣮ�ɹ�ѡ��������У�
�ٲ���������ƿ���ձ��ܽ�ͷ�ιܢ���Ͳ��������ƽ��ҩ�ף����������У�����ʱ����Ҫʹ�õ�������______������ţ�����ȱ�ٵ�һ��������______��
��1����Cu��ϡ���ᷴӦ��������ͭ��NO��ˮ�������ӷ�ӦΪ3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
��2��NO�����ʵ���Ϊ
=0.05mol����ͭ�����ʵ���Ϊx��
�ɵ����غ��֪��0.05mol����5-2��=x����2-0����
���x=0.075mol��
Cu������Ϊ0.075mol��64g/mol=4.8g��
�ɵ�ԭ���غ��֪����������ʵ���Ϊ0.075mol��2+0.05mol=0.2mol��
������Һ��HNO3�����ʵ���Ũ��Ϊ
=1mol/L��
�𣺸�������Һ��HNO3�����ʵ���Ũ��Ϊ1mol/L���μӷ�Ӧ��ͭ������Ϊ4.8g��
��3��Ũ��Һ������ϡ��Һ��Ҫ�ձ�������������ͷ�ιܡ���Ͳ������ƿ���ʲ���Ҫ�ڢޢߣ�
������500mL��ϡ���ᣬ��Ҫ500mL����ƿ���ʴ�Ϊ���ڢޢߣ�500mL����ƿ��
�ʴ�Ϊ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
��2��NO�����ʵ���Ϊ
| 1.12L |
| 22.4L/mol |
�ɵ����غ��֪��0.05mol����5-2��=x����2-0����
���x=0.075mol��
Cu������Ϊ0.075mol��64g/mol=4.8g��
�ɵ�ԭ���غ��֪����������ʵ���Ϊ0.075mol��2+0.05mol=0.2mol��
������Һ��HNO3�����ʵ���Ũ��Ϊ
| 0.2mol |
| 0.2L |
�𣺸�������Һ��HNO3�����ʵ���Ũ��Ϊ1mol/L���μӷ�Ӧ��ͭ������Ϊ4.8g��
��3��Ũ��Һ������ϡ��Һ��Ҫ�ձ�������������ͷ�ιܡ���Ͳ������ƿ���ʲ���Ҫ�ڢޢߣ�
������500mL��ϡ���ᣬ��Ҫ500mL����ƿ���ʴ�Ϊ���ڢޢߣ�500mL����ƿ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ