��Ŀ����
(14��)ij̽��С���ͬѧ���ʵ��̽������ˮ�����ķ�Ӧ����̽����Ӧ���������һϵ�����ʡ�
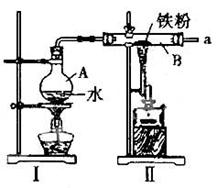
��1����ͼΪ����ˮ������Ӧ��ʵ��װ�ã�ʵ��ǰӦ�Ƚ��еIJ�����________________
________________ __��
��2��ͼIΪ����ˮ������װ�ã�ͼIIΪ����ˮ������Ӧ��װ�ã�д��Ӳ�ʲ�����B����������Ӧ�Ļ�ѧ����ʽ______ ________________________________��
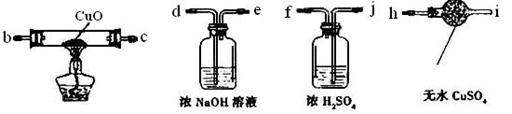
��3��Ϊ����֤Ӳ�ʲ�����B�з�Ӧ������������H2���������ͼ��ѡ���Ҫ��������ҩƷ����Ƴ�һ��װ�ã�������˳��Ϊ��a��_______��_______��_______��_______��________����������װ�õĽӿ���ĸ��
��4����Ӧ��ͼIIװ����Ӳ�ʲ�����B�ڵ�ȫ�����������Թ��У�����60mL1mol/L��ϡ���ᣬ����ǡ����ȫ�ܽ⣬�ٵμ�KSCN��Һ��������Һ����Ѫ��ɫ��д���˹��������п��ܷ�����Ӧ�����ӷ���ʽ__________ __��_________________________��________________________����ӦǰӲ�ʲ�����B�м���Fe�۵�����Ϊ__________g.

��1����ͼΪ����ˮ������Ӧ��ʵ��װ�ã�ʵ��ǰӦ�Ƚ��еIJ�����________________
________________ __��
��2��ͼIΪ����ˮ������װ�ã�ͼIIΪ����ˮ������Ӧ��װ�ã�д��Ӳ�ʲ�����B����������Ӧ�Ļ�ѧ����ʽ______ ________________________________��
��3��Ϊ����֤Ӳ�ʲ�����B�з�Ӧ������������H2���������ͼ��ѡ���Ҫ��������ҩƷ����Ƴ�һ��װ�ã�������˳��Ϊ��a��_______��_______��_______��_______��________����������װ�õĽӿ���ĸ��

��4����Ӧ��ͼIIװ����Ӳ�ʲ�����B�ڵ�ȫ�����������Թ��У�����60mL1mol/L��ϡ���ᣬ����ǡ����ȫ�ܽ⣬�ٵμ�KSCN��Һ��������Һ����Ѫ��ɫ��д���˹��������п��ܷ�����Ӧ�����ӷ���ʽ__________ __��_________________________��________________________����ӦǰӲ�ʲ�����B�м���Fe�۵�����Ϊ__________g.

��

��ϰ��ϵ�д�
 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
�����Ŀ

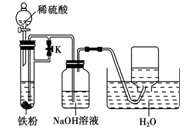


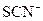 ����������ˮ����
����������ˮ���� �뵽CuSO4��Һ��
�뵽CuSO4��Һ�� 4H+ + O2��+ 2Cu
4H+ + O2��+ 2Cu ______________________��
______________________�� ��֮һ����ԭ����Fe3O4��4CO3Fe��4CO2������1��5 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����__________________��
��֮һ����ԭ����Fe3O4��4CO3Fe��4CO2������1��5 mol Fe3O4�μӷ�Ӧ��ת�Ƶ��ӵ����ʵ�����__________________�� a2Fe2O4+ NH3+ NaOH
a2Fe2O4+ NH3+ NaOH Fe3O4+4NaOH
Fe3O4+4NaOH ������1mol Na2Fe2O4���ɣ���Ӧ����____________mol����ת�ơ�
������1mol Na2Fe2O4���ɣ���Ӧ����____________mol����ת�ơ�