��Ŀ����
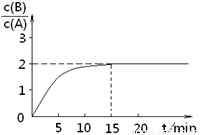
 ��֪��ij�¶�����2L�ܱ������м���һ����A���������»�ѧ��Ӧ��
��֪��ij�¶�����2L�ܱ������м���һ����A���������»�ѧ��Ӧ��2A��g���TB��g��+C��g������H=-48.25kJ?mol-1
��Ӧ������ʱ��t��A��BŨ������ͼ��ϵ������õ�15minʱ
c��B��=1.6mol?L-1�������н�����ȷ���ǣ�������
| A����Ӧ��ǰ15min��ƽ�����ʦͣ�A��=0.11 mol?L-1?min-1 | B��A�ij�ʼ���ʵ���Ϊ8 mol | C����Ӧ����ƽ��Ĺ����У��ų���������77.2kJ | D����ʹ��Ӧ����c��B��/c��A��=1.5�����º���ʱ������һ������A����ʵ�� |
��������ͼ��֪��15minʱ��Ӧ����ƽ�⣬ƽ��ʱc��B��=1.6mol?L-1��
=2����ƽ��ʱc��A��=0.8mol/L��
A������Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�������c��A����Ȼ�����V=
������ʣ�
B������Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȼ����c��A����A��ƽ��Ũ��+��c��A��=A����ʼŨ�ȣ��ٸ���n=cV���㣻
C������μӷ�Ӧ��A�����ʵ���������Ȼ�ѧ����ʽ����ų���������
D��Ҫʹ
=1.5��Ӧ�ı�����ʹc��B����С��c��A����������������ƽ��Ӱ�������
| c(B) |
| c(A) |
A������Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�������c��A����Ȼ�����V=
| ��C |
| ��t |
B������Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȼ����c��A����A��ƽ��Ũ��+��c��A��=A����ʼŨ�ȣ��ٸ���n=cV���㣻
C������μӷ�Ӧ��A�����ʵ���������Ȼ�ѧ����ʽ����ų���������
D��Ҫʹ
| c(B) |
| c(A) |
����⣺��ͼ��֪��15minʱ��Ӧ����ƽ�⣬ƽ��ʱc��B��=1.6mol?L-1��
=2����ƽ��ʱc��A��=0.8mol/L��
A��Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ʡ�c��A��=2��c��B��=2��1.6mol?L-1=3.2mol?L-1������ǰ15min��ƽ�����ʦͣ�A��=
=0.21mol?L-1?min-1����A����
B��Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ʡ�c��A��=2��c��B��=2��1.6mol?L-1=3.2mol?L-1��A����ʼŨ��Ϊ3.2mol?L-1+0.8mol?L-1=4mol?L-1����A�ij�ʼ���ʵ���Ϊ4mol/L��2L=8 mol����B��ȷ��
C���μӷ�Ӧ��A�����ʵ���Ϊ3.2mol/L��2L=6.4mol���ʷų�������Ϊ48.25kJ��
=154.4kJ����C����
D��Ҫʹ
=1.5��Ӧ�ı�����ʹc��B����С��c��A���������º���ʱ����������ǰ��ϵ��֮����ȣ�����һ������A��ѹǿ����ƽ�ⲻ�ƶ���c��B����c��A��ͬ��������ֵ���䣬��D����
��ѡ��B��
| c(B) |
| c(A) |
A��Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ʡ�c��A��=2��c��B��=2��1.6mol?L-1=3.2mol?L-1������ǰ15min��ƽ�����ʦͣ�A��=
| 3.2mol?L-1 |
| 15min |
B��Ũ�ȱ仯��֮�ȵ��ڻ�ѧ������֮�ȣ��ʡ�c��A��=2��c��B��=2��1.6mol?L-1=3.2mol?L-1��A����ʼŨ��Ϊ3.2mol?L-1+0.8mol?L-1=4mol?L-1����A�ij�ʼ���ʵ���Ϊ4mol/L��2L=8 mol����B��ȷ��
C���μӷ�Ӧ��A�����ʵ���Ϊ3.2mol/L��2L=6.4mol���ʷų�������Ϊ48.25kJ��
| 6.4mol |
| 2mol |
D��Ҫʹ
| c(B) |
| c(A) |
��ѡ��B��
���������⿼�黯ѧƽ��ͼ��ѧ���㡢Ӱ��ƽ������صȣ�ע��Dѡ���Ӧǰ�������������䣬����һ������A�������ƽ��״̬��ԭƽ��״̬Ϊ��Чƽ�⣬c��B��/c��A��ֵ���䣮

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
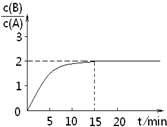
 ��֪��ij�¶�����2L�ܱ������м���һ����A���������»�ѧ��Ӧ��
��֪��ij�¶�����2L�ܱ������м���һ����A���������»�ѧ��Ӧ��2A��g��?B��g��+C��g������H=-48.25kJ?mol-1��Ӧ������ʱ��t��A��BŨ������ͼ��ʾ��ϵ������õ�15minʱc��B��=1.6mol?L-1�������н�����ȷ���ǣ�������
| A�����¶��´˷�Ӧƽ�ⳣ��Ϊ3.2 | B��A�ij�ʼ���ʵ���Ϊ4 mol | C����Ӧ����ƽ��Ĺ����У��ų���������154.4kJ | D����ʹ��Ӧ����c��B��/c��A��=3��ֻ�ܽ��ͷ�Ӧ�¶� |
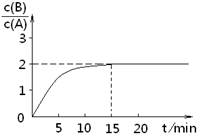
 B(g)+C(g)����H =-48.25 kJ
�� mol��1��Ӧ������ʱ��t��A ��BŨ������ͼ��ʾ��ϵ������õ�15minʱc(B)=1.6 mol��L��1�������н�����ȷ����
B(g)+C(g)����H =-48.25 kJ
�� mol��1��Ӧ������ʱ��t��A ��BŨ������ͼ��ʾ��ϵ������õ�15minʱc(B)=1.6 mol��L��1�������н�����ȷ����