��Ŀ����
���軯��ķ�ˮΣ�������ܶ�ɱ���������ˮ���CN-Ũ����0.01��0.04 mg��L-1ʱ����ǿ�ҵĶ�ɱ���ã����������軯��Ĺ�ҵ��ˮ�ķ���֮һ�ǣ�ʹCN-������ת��Ϊ�Ͷ���CNO-���䶾�Խ�Ϊ�軯���ǧ��֮һ����һ��ת��Ϊ�����Σ���NaCNO�������������ô������Σ�ͬʱ���ɽ�����������塣�����о��������ڹ�������£���ijЩ����С���������Һ����ɷ������Ե�������ԭ��Ӧ���ܿ콵�ⶾ�ԣ����ڶ������ѣ�TiO2�������Ͽ��Խ����軯����ж��
��1������������������Ӧ�е�������______________________________________________��
��2�������NaClO������NaCl�ķ�Һ��������������������Σ����Խ��⣩����д���˷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________��������N��Ϊ��3�ۣ�
�˷�Ӧ�б�������Ԫ����___________________________��
��3����������������������ý������������������Щ��
______________________________________________________________________________��
д�����ܷ��������ӷ���ʽ______________________________________________________��
��1������������������Ӧ�е�������______________________________________________��
��2�������NaClO������NaCl�ķ�Һ��������������������Σ����Խ��⣩����д���˷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________��������N��Ϊ��3�ۣ�
�˷�Ӧ�б�������Ԫ����___________________________��
��3����������������������ý������������������Щ��
______________________________________________________________________________��
д�����ܷ��������ӷ���ʽ______________________________________________________��
��1��������
��2��NaClO+NaCN="===NaCl+NaCNO " ̼����C��
��3��CO2��N2 2CNO-+2ClO-+H2O====2CO2��+N2��+2OH-+3Cl-
��2��NaClO+NaCN="===NaCl+NaCNO " ̼����C��
��3��CO2��N2 2CNO-+2ClO-+H2O====2CO2��+N2��+2OH-+3Cl-
�������ڸ���Ϣ����Ŀ�����������Ŀ�е���Ϣ�������ѧ�Ĵ������ε�֪ʶ����⡣

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
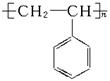 )�����������İ�ɫ��Ⱦ������Ϊͻ�����¹�ij��˾����������60�����н���ܵ���ɫʳƷ������������
)�����������İ�ɫ��Ⱦ������Ϊͻ�����¹�ij��˾����������60�����н���ܵ���ɫʳƷ������������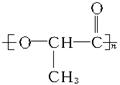 ��װ���ϡ�
��װ���ϡ�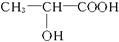 )���Դ���˷��͵���Һ����ȡ,ͨ�����۷�Ӧ�ɺϳɾ����ᡣ�ϳɾ�����Ļ�ѧ����ʽΪ____________________________________��
)���Դ���˷��͵���Һ����ȡ,ͨ�����۷�Ӧ�ɺϳɾ����ᡣ�ϳɾ�����Ļ�ѧ����ʽΪ____________________________________��